Thoughts, Epiphanies, Insights, & Quotables
Replies
-
My scale has both an ml and ml (milk) setting.
2 -
Yes. But the scale lies ;-)
It only has one real setting (grams) while telling you it has two by just showing the same value as either ml or g.
That said… apparently the world has moved on from when I was in grade school where they lied to me and had me convinced to this day that 1g of water at 4C / 39.2F is 1mL and that a meter (rod) is sitting in a display case in Paris for each and everyone of us to show up and compare it to our pocket tape measure! Reality appears to be more messy! :)
1 m = the metre is apparently now defined as the distance that light travels in vacuum in 1 / 299,792,458 of a second — my poor rod has been retired!And volume is totally derived!
1 m³ = a cube 1 m on a side.
1 L = 1 litre is defined as a 0.001 m³
1mL = 1 mililitre = 0.001 L
which makes it a cube that is 1cm x 1cm x 1cm
So by definition, 1 mL = 1 cm³ in volume.
And the mass (weight) of 1ml of water = density of water (at the temperature) x 1mL.Since density of water depends on temperature (plus small effects from pressure and water isotopes in the mix), we can loosely assume using the OLD definition that they sold me on, that at about ~4 °C, where water's density is very close to 1g/mL, it will be 1g.
But, in actual fact:
Temperature (°C) | Density (g/mL) | Mass of 1 mL (g)0 °C | 0.99987 | 0.99987 g
4 °C | 1.00000* | 1.00000 g
10 °C | 0.99973 | 0.99973 g
20 °C | 0.99821 | 0.99821 g
25 °C | 0.99704 | 0.99704 g
40 °C | 0.99224 | 0.99224 g
60 °C | 0.98320 | 0.98320 g
80 °C | 0.97180 | 0.97180 g
100 °C | 0.95840 | 0.95840 gValues in the table are (apparently) standard reference values for pure (air-saturated or air-free depending on table) water at ~1 atm
Around 1795 the liter was linked to 1 kg of water (at its temperature of maximum density), so people expected 1 L ≈ 1 kg.
- Small experimental differences and the difficulty of reproducing exact conditions caused a tiny mismatch between “1 L = 1 kg of water” and the geometric cube definition.
In the 20th century the liter was fixed as exactly 1 cubic decimeter (1 L = 1 dm³ = 0.001 m³) to avoid those experimental problems; the water-mass relation remains only an approximation that depends on temperature.
Item Density (g/mL) Notes
Cotton candy 0.002–0.005 Basically spun sugar air
Popped popcorn 0.05–0.15 Very low density popped starch
Canned whipped cream 0.30–0.40 Mostly air, propellant expands it
Non-dairy whipped topping (thawed) 0.35–0.45 Oil-based plus air
Freshly whipped dairy cream 0.45–0.55 Doubles in volume after whipping
Cheap “airy” ice cream 0.55–0.65 High overrun, more air
Premium ice cream 0.85–0.90 Low overrun, more solids/fat
Butter (soft) 0.90–0.94 High fat content, low water
Olive oil 0.91–0.93 Less dense than water, will float
Canola oil 0.92–0.93 Similar to olive oil, will float on water
Skim milk (4 °C) 1.033 Higher density from dissolved solids, no fat
2% milk (4 °C) 1.031 Slightly less dense than skim milk
Homogenized whole milk (4 °C) 1.030 Lower density from higher fat content
Water (20 °C) 0.99821 Standard density at 20 °C, 1 atm
Cream cheese 1.05–1.10 Solid dairy plus water
Peanut butter 1.10–1.18 Fat and solids packed densely
Sweetened condensed milk 1.28–1.33 Reduced water, high sugar
Maple syrup 1.33 Dense sugar solution
Corn syrup 1.36–1.40 Glucose syrup, high solids
Honey 1.42 Mostly sugars, low water content
Molasses 1.40–1.45 Very high dissolved solids
So 140g to 145g of molasses is 100ml. And 30g to 40g of canned whipped cream is also 100ml. And the poor scale has no way of figuring this out!0 -
Ain’t nobody got time for all that!
2 -
Lol, i still don't understand, nor have a feel/sense of the values for the system… like when weather expressed in C, or people post weight in kg…
I do understand a 2 liter pop bottle though... :D
2 -
Hmmm…. Ms Yooly… and how would you then know that when you are considering logging your two tbsp of strawberry jam… that you could probably get 38 to 39g of strawberry jam as opposed to 30g out of the jar? Are you saying you would leave behind the delicious 9g I would be consuming!?!?!??! 😲
1 -
I have never acquired or cultivated the talent of accidentally UNDER serving myself. 😂
1 -
Hahaha, me neither!
1 -
Technology 0.25 alpha — farm it out and let the poor slobs sort it out… and tell them it is a "premium" feature!
What am I talking about? Photo logging via "another" app ;-)100% success rate in identifying the ingredients (if you accept white bread instead of rye bread)
and 100% success rate in identifying the quantities at 50% of LESS!
🤣So my sandwich had half the bread, half the filling, half… reminds me of logging jam by grams ;-)
Off to the back burner it goes!
1 -
Yeah, those adds for logging by photo kill me. Eyeballing is notoriously inaccurate, which is the whole point of measuring, and weighing, which is more accurate yet. That photo can't tell if you used 1/2 cup of butter and 1/2 cup of heavy cream in your pasta sauce vs 1 tbsp butter, 2% milk and cottage cheese for example. Plus, sizes/amounts.
2 -
Ditto that...
However... to my utter surprise, I have found a nudge of reality when I snap a pic.
At my last docs 6m apptmt, Doc delved into some weight mgmt q & advice, so I showed him my 'myplate' meals, and we had a really nice conversation. It added reality to what was just a typical advice conversation.
After that, it has been just part of eating now. Then noticed it has helped me stick to my meal parameters better - especially recently when the honeymoon wore off. The good news, I didn't quit. Still snapping meal pics.
Btw, love seeing other peoples pics too.
1 -
Sat mornings I putter with tv in background, often on food factory/cooking shows and snag interesting ideas. Today...
- For a butter chicken jar sauce, they warmed some oil, added (middle eastern cuisine type) spices to pre-cook a bit before adding tomato sauce & paste. Simmered a bit then added the cardamon & garam marsala (cinnamon, allspice, nutmeg) type spices, simmer more. 20min b4 done, add fresh cut herbs (cilantro.)
- In a prior show of some sort, suggested adding a bit of molasses to mellow the spices, and beyond tomatoes, add citrus/vinegars for the acid balance.
Another episode on some candy making - the guy hand mixing said (paraphrased) - 'you have to understand what sugar can do, then you can make it do what you want.'
- Epiphany, uh duh... seek info, understand, deploy...
- For me, transferred beyond cooking to a fallout of yummy cooking, when i eat excess yummy cooked foods, it triggers voracious appetite.
- Learning/understanding my particular threshholds and particular trigger foods so I can help myself is simply an ongoing process… (& ongoing awareness required because my triggers may shift...)
- I can't bear to think about forever rules, but I can tackle decisions for today/a few days batch food shopping/cooking.
- Tomorrow will be here soon enough, I can call those 'eat food' balls then.
1 -
I did do a different picture a couple of days later. This new picture was a good conversation starter ;-) did it get everything right? Not even remotely. But it did prompt me to consider some of the quantities I was using both up and down and with some editing and additions I did come up with what I thought was a reasonable log of the meal. (mongolli grill, so obviously NOT an easy picture to log from)
1 -
I could see if the app had the actual nutrition info from the source AND a calibrated picture of the amount in the picture, then a good interpretation would be possible.
Otherwise, not inclined to trust the interpretation as completely accurate.
However, a decent estimator when eating something with unknown nutrition? Thinking a potluck or buffet line... What if the app also asked about likely deconstructed ingredients? Could be helpful...
Afterall, this is how the hand equivalent guestimators are helpful.
There are life impracticalities of 100% true tracking situations, where we need a practical way of estimating our food choices.
After working with the rd this year starting with logging calorie counts in conjunction with the myplate swapoable food exchanges - think that has led to a practical understanding/process for us.
In sept, we will explore anti-inflammatory and fodmaps, along with a pre-bariatric wls effort...
It all was intimidating at start, but have gained some confidence in food choices by looking at it from these various lenses. Really rather surprised by it.
A picture indeed is worth a 1000 words, or at least to help me guestimate.
2 -
BClady, Laurie, Creamie- where art thow!
1 -
Had a bone scan and 1 of the food ideas was dried figs. Surprised me so did a quick AI search on the nutrition and health benefits and adding them to my rotation of dried prunes & cranberries/craisins.
1 -
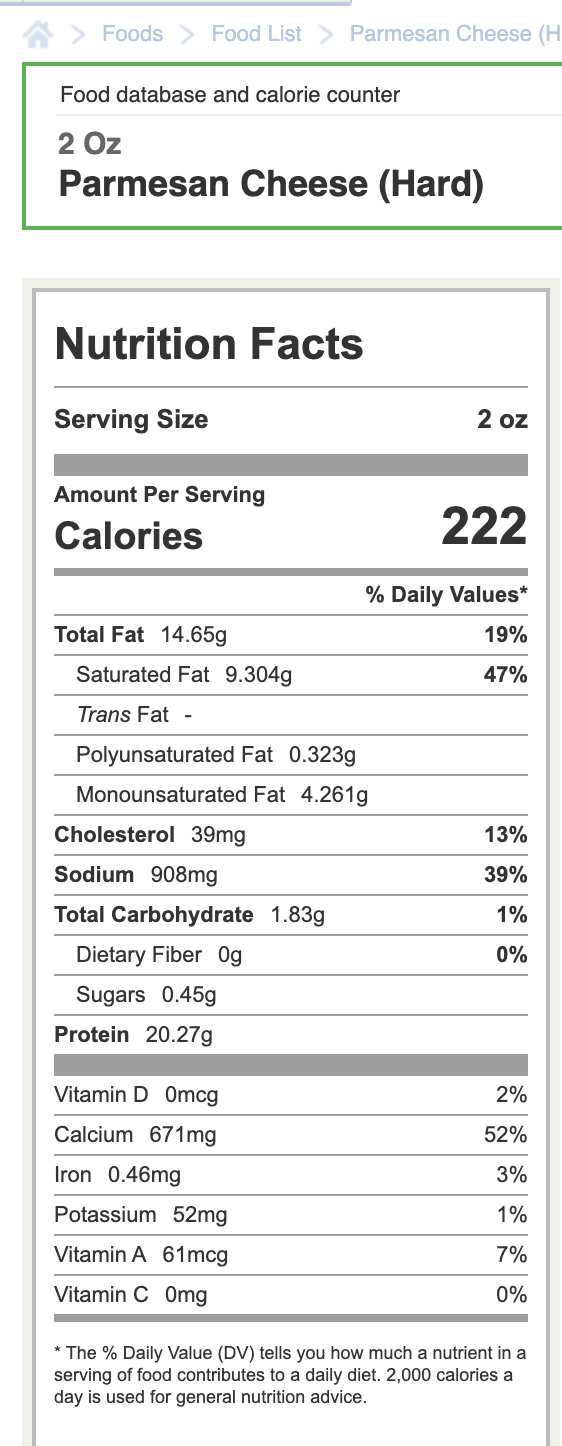
Struck my funny bone (saw this posted elsewhere.)
A high protein snack bar…
2 -
I confirm your numbers! Hard grated has less protein which surprised me!
1 -
0
-
Fiber minimal, so pair with something fiberiffic... ;)
Today's affirmative…if ya ain't dead, today is another chance to try, to do, to live more - live it happy. ♡
1 -
I like your post was thinking of the dude really feel like saying ditch the job or ditch the house and move closer and wake up to the fact that neither is useful to you once you're ashes... but I am concerned it might be a bit much 🤯🤷🏼♀️🤦♂️ not very mr sunshine here!
0 -
Yep... not much in tge mood at the moment for wallowing & pity parties ;)
(Don't mean to sound uncaring, but I don't think indulging is the wqy to show care.)
Every day is bonus...
2 -
Just putting a marker - have been eating mostly homecooked from scratch w/o much added salt nor sugars.
Surprised the strawberry swirled cheesecake at dinner was super almost too sweet to me.
That's a 1st... noticed the sweetness but... tongue still did the yippy skippy!
2 -
If you gotta elevate your sore swollen arthritic/ankle tendonitis foot - do it on a cruise ship!
HEY I was able to actually edit this post. 🎉 🎉🎉🎉🎉🎉🎉🎉🎉🎉
1 -
Oh the view! Hear ya on elevating.
Edit working here too, yay :)
1 -
Nice view!!!🤣🤣🤣
1 -
1
-
2
-
Tues is our last RD consult. It has been a thought provoking year considering food choices and meal building. I will miss that guided focus.
Not sure what to explore next.
2 -
Looks like someone or something has hacked and spammed all the community forums! No point going there until it’s cleaned up 🧹
0 -
Ikr! Crazy.
1