What is a safe sugar alternative ?
Options
Replies
-
I enjoy using Monk Fruit In The Raw packets.
 0
0 -
I am a weirdo who doesn't like the taste of sugar in my tea, but I do like slightly sweetened tea, so I use stevia drops. It doesn't taste like sugar, but it is sweet. You'll either love it or hate it, and I love it, personally.0
-
If you can cut back on the sugar so that you use just a little, that's a good way to go. I use Stevia--it's been tested extensively in Germany where they have a commission to approve over-the-counter stuff. Artificial sweeteners may not cause cancer, but that does NOT mean they are good for you. I personally suffer from neuropathy in my feet because of aspartame (average less than 2 packs a day). Lots of other people have reported nerve damage, too. I switched to Splenda (same less than 2 packs a day) and my bad cholesterol hit 249 in less than a year (should be less than 100). My doctor just told me that other doctors are seeing the same problem with their patients. And, yes, there is absolutely no doubt that these things were the cause.0
-
tedboosalis7 wrote: »Don't use artificial it's gross. Made by chemical manufacturers, some of whom made Agent Orange. Couldn't get their stuff approved for years until they got their own employees working in the regulatory agencies - seriously, why eat something that was made by the same manufacturers of war-time chemical weapons? Stevia is fine.

This needs to be stickied again:Aaron_K123 wrote: »Hey everyone. I've seen my fair share of posts on the forums with regards to the dangers of aspartame and how it is a poison or a toxin or a carcinogen. Wanted to clear some things up about aspartame if I could just to explain why I personally believe there is absolutely no reason to fear aspartame.
What is aspartame?
For my fellow biochemists just simply saying its a methylester of phenylalanine and aspartate is enough to answer that question but figure I should take the time to explain what that means. Phenylalanine and aspartate are 2 of the 20 naturally occuring amino acids found in all protein. As our sequence information databases grow we know more and more about what the average amino acid composition of proteins is. Here is a download of our sum total sequence information from protein from the UniProt database. http://web.expasy.org/docs/relnotes/relstat.html Section 6 shows the amino acid frequencies which shows phenylalanine (Phe, F) at 3.6% of protein and aspartate (Asp, D) as 5.46%. This information will come in handy later. Amino acids are connected to one another naturally via a peptide bond between the carboxylic acid group and the amino group of each individual amino acid. Aspartame is simply a dipeptide of phenylalanine and aspartate where the terminal carboxy group is substituted for a methylester.
All amino acids have the following structure:
Aspartame's structure is this: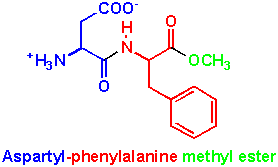
And the natural dipeptide between aspartate and pheynlalanine (aspartyl-phenylalanine) is this:
Aspartame's structure is just a natural dipeptide of phenylalanine and aspartate where the terminal carboxylic acid group has been methylated on the oxygen to form a methyl ester so instead of COO- it is COCH3. That is the only difference.
What happens to aspartame when we ingest it?
As with any protein aspartame is hydrolized in the stomach acid and metabolically broken down in the intestine to the breakdown products of aspartate, phenylalanine and methanol in a weight ratio of 4:5:1. What that means is that 10mg of aspartame will be broken down in your body to 4mg of aspartate, 5mg of phenylalanine and 1mg of methanol before it enters your blood. [citation: http://informahealthcare.com/doi/abs/10.1080/10408440701516184]. No aspartame enters your blood intact.
How much of each metabolite do you get from ingesting one diet soda?
So the metabolic products of aspartame are aspartate,phenylalanine and methanol in a 4:5:1 ratio. One can of diet coke has about 180mg of aspartame. That means it is broken down to 72mg of aspartate, 90mg of phenylalanine and 18mg of methanol.
How much of those metabolites are in other foods?
As mentioned phenylalanine and aspartate are naturally occuring amino acids found in all proteins. Protein is about 5.46% aspartate and about 3.6% phenylalanine on average. So let us say you have a 4oz piece of chicken breast. A small 4oz chicken breast has about 24g of protein. That means that in that chicken breast there is .036*24*1000 = 864mg of phenylalanine and .055*24*1000 = 1320mg aspartate. That means to get the same amount of aspartate and phenylalanine from diet coke as you do from one 4oz chicken breast you would have to drink 18 diet cokes. In my diet I eat around 180g of protein in a day which means to equal the amount I get from my normal diet of whole foods I would have to drink 135 cans of diet coke.
Methanol is a biproduct of all fermentations. As such it is present in things that ferment, including things that are in the process of ferminting whether we think of them as alcohol or not. That means things like fruit. So how much methanol is present in 1 8oz glass of orange juice for example? Well according to this study of presence of methanol in a variety of orange juices [citation: http://archive.food.gov.uk/maff/archive/food/infsheet/1993/no17/table1.htm] the amount of methanol averages around 125 mg/kg. 8oz is 0.23kg so that means that 8oz of orange juice has about 29mg of methanol in it. Recall that a diet soda the aspartame content would break down to about 18mg of methanol. In otherwords orange juice, or really any fruit juice, has more methanol in it per oz than soda.
Conclusion
We know what aspartame is, we know its structure, we know its composition and we know exactly what happens to it in the human body. We are very familiar with the metabolic breakdown products of phenylalanine, aspartate and methanol all of which are found in higher amounts in natural whole foods such as fruits and proteins. There is no reason at all to suspect that aspartame presents any sort of toxic or carcinogenic risk from the chemistry of the molecule and indeed toxicology studies of aspartame in humans show no toxic dose level [citation: http://informahealthcare.com/doi/abs/10.1080/10408440701516184]. Stories of the toxicity of aspartame are heresay, anecdotal and fear mongering and are not supported by either the chemistry, the biochemistry, the toxicology or the epidemiology.
Yet online on the internet we get stuff like this:
Sensationalistic irrational garbage.
http://community.myfitnesspal.com/en/discussion/1308408/why-aspartame-isnt-scary/p1
0 -
liekewheeless wrote: »Depends on what you use it for.
I like to use stevia (the leaf) in my tea. Makes sense to me, it's natural and it steeps with the tea.
I don't like baking with sugar substitute. I haven't found anything that work for me.
I've actually had good success replacing half the sugar in my baked goods w/stevia. Don't even notice it. But I definitely taste it in beverages. Go figure!
0 -
Do you have a medical issue that requires you to reduce sugars in your diet? For weight loss only, calories are what are important not how much sugar you eat.
If I were you, I would look at your food diary and see where most of the sugar is coming from. You might just make a simple change without needing to use sugar substitutes. Maybe reducing the consumption of sweetened drinks, pre-made foods or baked goods in your diet is a better plan. Try that for a month or two and I bet you won't even want as much sugar.
.
Agree with this. When I started on MFP, I had it in my head that maybe I needed to eliminate sweets. When I realized your food log tracks sugar, I was surprised to see that my sugar intake really wasn't as bad as I thought it was and that a few simple adjustments kept me under the default level. I still have treats and real sugar (though I'm experimenting more with stevia and buy yogurt w/splenda), but don't seem to have the constant cravings for it I used to.
0 -
The safest bet is to stop the use of all sweeteners. We all know that over use of sugar is not good, what we don't know is what over use of artificial sweeteners will do. My thought is that tricking your body or taste buds that something artificial is sweet can't be good. I always see "all natural" but I am not impressed because the same could be said about arsenic. The bottom line is you can train yourself to not need sugar in iced tea and coffee. All other sweet drinks like soda are empty calories and can be eliminated. I get enough sugar from fruits without going to the sugar bowl.0
-
By the way, to get around the FDA stringent approval processes for sugar subtitutes, Stevia is marketed as a food supplement. It has not been tested as extensively as the others. So it's long term effects is unknown.
I don't have any idea how long it has been since getting sugar from sugar cane has been done, but using Stevia leaves has been done for many thousands of years. It has passed the test of time which is far more indicative of safety than a test done in a lab or on a small study group.
0 -
beemerphile1 wrote: »By the way, to get around the FDA stringent approval processes for sugar subtitutes, Stevia is marketed as a food supplement. It has not been tested as extensively as the others. So it's long term effects is unknown.
I don't have any idea how long it has been since getting sugar from sugar cane has been done, but using Stevia leaves has been done for many thousands of years. It has passed the test of time which is far more indicative of safety than a test done in a lab or on a small study group.
Actually I would argue the opposite. For centuries, the Romans would boil down grape leaves into a sweet liquid (defrutum) that was used in all sorts of ancient recipes and for food preservation. Unfortunately, there's evidence that the resultant liquid often contained lead acetone which we now know to be poisonous. The Roman's inability to isolate and individually test their food additives like we do today in a lab, led them to the false belief that defrutum is OK.
The scientific method is a far more useful tool to determine precisely what is or isn't deleterious than an appeal to what was done in the past.0 -
I drink my tea and coffee without any sweetener at all. Sometimes I add some lemon or lime in iced tea. My doctor recommended that I quit using artificial sweeteners to see if it helped my arthritis and it turned out that it did so I avoid them now. Prior to that I did enjoy Sweet n' Low but always hated Equal and especially Splenda. At first I used stevia and monk fruit in the raw but I didn't love the flavor they imparted in my tea so I just stopped sweetening it all together and now I enjoy it plain.
Honestly if you can get by with a teaspoon of real sugar per drink I'd go with that. That's not a bad amount at all!0
Categories
- All Categories
- 1.4M Health, Wellness and Goals
- 392K Introduce Yourself
- 43.6K Getting Started
- 259.8K Health and Weight Loss
- 175.7K Food and Nutrition
- 47.4K Recipes
- 232.3K Fitness and Exercise
- 403 Sleep, Mindfulness and Overall Wellness
- 6.4K Goal: Maintaining Weight
- 8.5K Goal: Gaining Weight and Body Building
- 152.8K Motivation and Support
- 7.9K Challenges
- 1.3K Debate Club
- 96.3K Chit-Chat
- 2.5K Fun and Games
- 3.4K MyFitnessPal Information
- 23 News and Announcements
- 999 Feature Suggestions and Ideas
- 2.4K MyFitnessPal Tech Support Questions






