Biochemistry answers for common weight loss questions: What are Macros-Fats

Aaron_K123
Posts: 7,122 Member
Macros, in nutrition, are the primary different types of molecules within your food that you derive energy from: protein, fats and carbohydrates. In biochemistry these three types of molecules are the main molecules that make up all of biological life. I will go through each one and explain what their general composition is, what role they play in life and what they are broken down into when we metabolize them for energy. Lets start with fat.
Fats
Fats in biology take the form of fatty acid chains which are long chains of carbon with only hydrogen molecules attached to fill their valence shell. These types of molecules are called hydrocarbons (hydrogen + carbon). Hydrocarbons you might be familiar with are methane (natural gas), propane, butayne which are different only in how long the chain of carbons is.

Gasoline is just a mixture of different chain length hydrocarbons between 4 and 12 carbons long. Hydrocarbons alone are not soluble in water (they are called hydrophobic) and we are not capable of digesting or processing them (don't drink gasoline folks). On a side note you could also get the smaller version of these molecules to be polar and soluble in water (hydrophilic) by just replacing one of the hydrogens with oxygen. The result would be that methane would become methanol and ethane would become ethanol, which you might be familiar with as alcohols.
In biology the ends of long hydrocarbon chains are modified with a carboxylic acid which is carbon with attached oxygens , this introduces a polarity to the molecule that gives one end a bit of reactivity.

Typically in living organisms for storage of hydrocarbons these molecules are attached via their reactive carboxylic acid group to glycerol in order to make them semisoluble. Three chains can be attached to glycerol in what is called a triglyceride.
Glycerol:

Triglyceride

If the carbons in the hydrocarbon chain are linked only with single bonds then they are referred to as being saturated. A saturated fat is a triglyceride where all the hydrocarbon chains are single-bonded. An effect of this is unsaturated fats are straight and can pack very tightly, they will form solids at room temperature like butter. An unsaturated fat is where a double bond is introduced between some of the carbons. That double bond can either have hydrogens connected to the joined carbons on the same side (cis) on opposite sides (trans). If it is cis then a kink is introduced into the chain, if it is trans the chain remains straight.

As a result cis unsaturated fats with that kink cannot pack tightly together and as a result they will form liquids at room temperature (oils) rather than solids. Trans-fats however can still pack tightly together so they behave more like saturated fats.
Triglycerides are how hydrocarbons are stored in your body as fat but arguably this is not the most important role fats (hydrocarbons) play in your body. Aside from energy storage fats play a critical role in life. Another type of fat is a glycerol molecule (2) where two fatty acid chains are attached (3) as well as a very polar (hydrophilic) molecule called phosphate (1). These molecules are called phospholipids.

The phosphate head group of the phospholipid wants to be near water while the hydrocarbon tails do not. As such when phospholipids are put into water and stirred up they form layers where the hydrocarbon tails pack on the inside and the phosphate heads line up on the outside facing the water. This bilayer is what makes a cell. Living cells use these layers as basically skin to separate their insides from the outside. Without these fat molecules you couldn’t have life.

(in this image the polar glycerol-phosphate is the red circle while the hydrocarbon chains are the two squiggly lines)
Thinking about fats in terms of macros though we are thinking about them as sources of energy so we are talking about triglycerides. When utilized for energy those fatty acid chains are broken down in an oxidation reaction that combines oxygen with the hydrocarbon chain to break it apart releasing energy and forming carbon dioxide and water.
Methane being the simplest hydrocarbon the reaction would look like this:
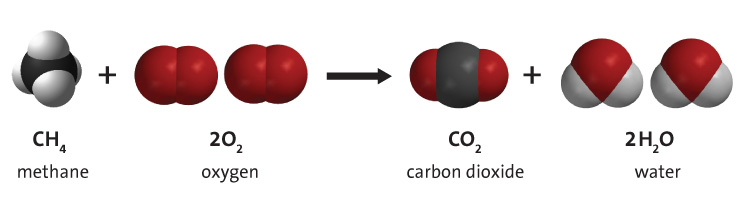
This reaction is carried out by enzymes in your body. Each step releases some energy which is either captured in energetic bonds of adenosine triphosphate (ATP) molecules (lifes universal energy currency molecule) or released as heat. You breath in oxygen to run the reaction and breath out the waste product carbon dioxide.
As you will begin to see as complex as the biochemical pathways are for the metabolism of the wide variety of potential food molecules they all boil down for all macros into this basic oxidation reaction: Energy molecule + oxygen goes to Carbon dioxide plus water plus released energy.
This reaction, by the way, is the exact same oxidization reaction carried out when you light something on fire and it burns. The energy in that case comes out as heat. In fact one way of measuring the energy content of a fat molecule (or any energy molecule) is to burn it in a contained vessel and measure how much energy is released by burning it within that vessel and using the trapped heat to heat up a known quantity of water. The amount of energy can needed to raise the temperature of one liter of water by one degree Celsius has a name, it is called one Calorie.
Fats (triglycerols) have a energy density of approximately 9 Calories for every gram of fat. What that means is if you burn 1 gram of fat and capture all of the energy to heat water you could heat 9 liters of water by one degree celsius (or 1 liter of water by 9 degrees celsius). So in terms of nutrition and energy fat you consume that is used for energy production can be thought of as a macro that gives 9 Calories for every gram you consume. That energy is mostly captured in ATP molecules that can then be used to help power the various functions of your body. The remaining energy released as heat helps keep your body running at the warm 37 degrees celsius it requires to function.
Fats
Fats in biology take the form of fatty acid chains which are long chains of carbon with only hydrogen molecules attached to fill their valence shell. These types of molecules are called hydrocarbons (hydrogen + carbon). Hydrocarbons you might be familiar with are methane (natural gas), propane, butayne which are different only in how long the chain of carbons is.

Gasoline is just a mixture of different chain length hydrocarbons between 4 and 12 carbons long. Hydrocarbons alone are not soluble in water (they are called hydrophobic) and we are not capable of digesting or processing them (don't drink gasoline folks). On a side note you could also get the smaller version of these molecules to be polar and soluble in water (hydrophilic) by just replacing one of the hydrogens with oxygen. The result would be that methane would become methanol and ethane would become ethanol, which you might be familiar with as alcohols.
In biology the ends of long hydrocarbon chains are modified with a carboxylic acid which is carbon with attached oxygens , this introduces a polarity to the molecule that gives one end a bit of reactivity.

Typically in living organisms for storage of hydrocarbons these molecules are attached via their reactive carboxylic acid group to glycerol in order to make them semisoluble. Three chains can be attached to glycerol in what is called a triglyceride.
Glycerol:

Triglyceride

If the carbons in the hydrocarbon chain are linked only with single bonds then they are referred to as being saturated. A saturated fat is a triglyceride where all the hydrocarbon chains are single-bonded. An effect of this is unsaturated fats are straight and can pack very tightly, they will form solids at room temperature like butter. An unsaturated fat is where a double bond is introduced between some of the carbons. That double bond can either have hydrogens connected to the joined carbons on the same side (cis) on opposite sides (trans). If it is cis then a kink is introduced into the chain, if it is trans the chain remains straight.

As a result cis unsaturated fats with that kink cannot pack tightly together and as a result they will form liquids at room temperature (oils) rather than solids. Trans-fats however can still pack tightly together so they behave more like saturated fats.
Triglycerides are how hydrocarbons are stored in your body as fat but arguably this is not the most important role fats (hydrocarbons) play in your body. Aside from energy storage fats play a critical role in life. Another type of fat is a glycerol molecule (2) where two fatty acid chains are attached (3) as well as a very polar (hydrophilic) molecule called phosphate (1). These molecules are called phospholipids.

The phosphate head group of the phospholipid wants to be near water while the hydrocarbon tails do not. As such when phospholipids are put into water and stirred up they form layers where the hydrocarbon tails pack on the inside and the phosphate heads line up on the outside facing the water. This bilayer is what makes a cell. Living cells use these layers as basically skin to separate their insides from the outside. Without these fat molecules you couldn’t have life.

(in this image the polar glycerol-phosphate is the red circle while the hydrocarbon chains are the two squiggly lines)
Thinking about fats in terms of macros though we are thinking about them as sources of energy so we are talking about triglycerides. When utilized for energy those fatty acid chains are broken down in an oxidation reaction that combines oxygen with the hydrocarbon chain to break it apart releasing energy and forming carbon dioxide and water.
Methane being the simplest hydrocarbon the reaction would look like this:

This reaction is carried out by enzymes in your body. Each step releases some energy which is either captured in energetic bonds of adenosine triphosphate (ATP) molecules (lifes universal energy currency molecule) or released as heat. You breath in oxygen to run the reaction and breath out the waste product carbon dioxide.
As you will begin to see as complex as the biochemical pathways are for the metabolism of the wide variety of potential food molecules they all boil down for all macros into this basic oxidation reaction: Energy molecule + oxygen goes to Carbon dioxide plus water plus released energy.
This reaction, by the way, is the exact same oxidization reaction carried out when you light something on fire and it burns. The energy in that case comes out as heat. In fact one way of measuring the energy content of a fat molecule (or any energy molecule) is to burn it in a contained vessel and measure how much energy is released by burning it within that vessel and using the trapped heat to heat up a known quantity of water. The amount of energy can needed to raise the temperature of one liter of water by one degree Celsius has a name, it is called one Calorie.
Fats (triglycerols) have a energy density of approximately 9 Calories for every gram of fat. What that means is if you burn 1 gram of fat and capture all of the energy to heat water you could heat 9 liters of water by one degree celsius (or 1 liter of water by 9 degrees celsius). So in terms of nutrition and energy fat you consume that is used for energy production can be thought of as a macro that gives 9 Calories for every gram you consume. That energy is mostly captured in ATP molecules that can then be used to help power the various functions of your body. The remaining energy released as heat helps keep your body running at the warm 37 degrees celsius it requires to function.
9
Replies
-
For those who might point out that I didn't actually explain how fats are broken down within our body in any sort of detail understand that that was on purpose. I'm trying to give the forum-level post sized overview and to do so explaining the decomposition of macros as an oxidation reaction that releases heat, energy, carbon dioxide and water is accurate on a big-picture level. Considering even the basics of the actual biochemical pathways themselves take an entire year long upper division college course to explain to people I think it would be silly of me to even try.
For those who want to dive into that ocean and paddle about here is an interactive pathway map of biochemistry as we currently understand it : http://biochemical-pathways.com/#/map/1
It is searchable, you just have to hit that little blue arrow on the left of the page. Fatty acid oxidation pathways are in section E8 of the map. Ketone bodies are right next door in F8 for all you keto people. Knock yourself out.
3 -
Thanks for a quick revision of the old biochemistry text book.
My comments on this post which might be relevant if you plan further posts on other macros.
- while explaining a bit about fats in general, and their molecular structure there is little information here about oral fat consumption vs fat storage
- there is also very little about the function of fats in bodily functions such as hormone production
- I’m not sure methane is the most relevant example, despite relative simplicity in regard to expressing the energy equation
- I feel like if you are going to explain the Kreb’s cycle and the role of APT this could be simplified with a more specific focus on this and a specific diagram of this process.
- you talk about the bonds and properties (liquid vs solids) at room temperature and the different bonds of different fat molecules, but not how this effects the human body
................
Don’t get me wrong, your info is accurate but personally I don’t feel it’s putting a focus on the macro of fat and relevance to people’s dietary intake and why they need to get enough of this particualar macro nutrient.
................
2 -
This content has been removed.
-
@lizery Yeah all valid criticisms thank you. I suppose the more accurate title for this would be "Stuff I know about fats from biochemistry that I know well enough to describe in my own words without having to look things up that I think might be of interest to people here"
Going through your points:
I assume you mean how the body decides what it is going to do with a consumed fat molecule, whether it metabolizes it on the spot, sends it for use as a biomolecule or sends it for storage in adipose tissue. Admittedly I don't remember off the top of my head so I didn't go into it, don't want to say something I'm not 100% confident in and have it turn out to be wrong.little information here about oral fat consumption vs fat storage
I didn't want to only talk about fat as energy because I was worried that would give the impression that was all it was used for, so I gave the one example I thought was easiest to understand and arguably most central to life which is membranes. Yeah there are lots of uses for fat but I didn't want to just list them all off.there is also very little about the function of fats in bodily functions such as hormone production
Yeah our bodies don't actually digest methane gas so no not the most directly relevant. I just wanted to explain the reaction balance in the simplest terms possible and not giving a real world example as I figured that would just be more confusingI’m not sure methane is the most relevant example, despite relative simplicity in regard to expressing the energy equation
This I don't really agree with. I think if I went into acetyl-CoA as the branchpoint and entry into the Kreb cycle and then mentioned the Kreb cycle and electron shuttling to the electron transport chain for ATP generation it would have been way to complicated and it either would have been a rain of jargon no one understood or if I took the time to explain it I'd be posting something way way to long (which it probably already is). I felt like I had to mention what happened to the energy from the reaction, that it was stored in an "energy currency" molecule so I mentioned it but didn't go into any depth.I feel like if you are going to explain the Kreb’s cycle and the role of APT this could be simplified with a more specific focus on this and a specific diagram of this process.
I guess that was just in the "neat fact" category of my memory, I know what a trans-fat is but I'm not off the top of my head knowing why that is particularly bad for us. As for unsaturated and saturated I could get into how the mix of them plus cholesterol can be used to modulate the fluidity of membranes but that seemed way off topic.you talk about the bonds and properties (liquid vs solids) at room temperature and the different bonds of different fat molecules, but not how this effects the human bodyDon’t get me wrong, your info is accurate but personally I don’t feel it’s putting a focus on the macro of fat and relevance to people’s dietary intake and why they need to get enough of this particualar macro nutrient.
You are probably right that it was not on topic enough and too detailed to really be useful. Ended up just being me rambling about stuff I think is neat to know.
I guess I feel like if you know what fat is and what its uses in our body is then it demystifies it enough where its no longer this scary thing to be avoided. I think to the majority of people fat is this "ick" thing that clogs arteries and has way to many calories and, well, makes you fat.4 -
-
This content has been removed.
-
3
-
Your recent post about salt and water was helpful and informative. I imagine this one will be too, to somebody. As my third-grade teacher explained to mom and dad, education isn't for everybody.2
-
This content has been removed.
-
I'm rapidly becoming a nerd-girl fan. Anything you feel motivated to type is fine by me. Others may differ, and have the option of skipping to the next post.4
-
I'm a fan of these posts. I may not fully grasp the whole thing as chemistry isn't my strong suit, but I like the challenge of trying to follow along.2
-
Aaron_K123 wrote: »
Ignore the haters. Some of us enjoy understanding how and why things happen as they do.
6 -
Oh yeah feel free to pick on them I'm not shying away from criticism. I just think people might be right that this time I crossed a line into being overly technical.
If I post on a topic not many people know about but is at a level that is engaging and a topic that interests people on the forums then it could be useful and informative for the community. If I post on a topic not many people know about at a level that is not engaging and on something that borders on off-topic then it won't be that useful. Its easy to cross that line if one gets technical unfortunately. You risk going to a level of detail where people reading it either already know it or they don't care to know it.1 -
collectingblues wrote: »Aaron_K123 wrote: »
Ignore the haters. Some of us enjoy understanding how and why things happen as they do.
The posts I've gotten so far haven't been haters, they brought up some valid criticisms...I'm okay with that. If you want to teach something its appropriate to word it at the right level and if you don't you can't just blame the audience, its ones own responsibility to come up with a way to present in a way that is engaging. I'm not discouraged I just think they might have a point. If this post ends up being popular I'd continue the series but I think they might be right that it is too technical. If that is the case probably won't bother on the other macros and just do something else.0 -
Your recent post about salt and water was helpful and informative. I imagine this one will be too, to somebody. As my third-grade teacher explained to mom and dad, education isn't for everybody.
Oh yeah trust me, I'm not under any illusions that interest in stuff like this will be niché at best.0 -
Aaron_K123 wrote: »
Oh yeah feel free to pick on them I'm not shying away from criticism. I just think people might be right that this time I crossed a line into being overly technical.
If I post on a topic not many people know about but is at a level that is engaging and a topic that interests people on the forums then it could be useful and informative for the community. If I post on a topic not many people know about at a level that is not engaging and on something that borders on off-topic then it won't be that useful. Its easy to cross that line if one gets technical unfortunately. You risk going to a level of detail where people reading it either already know it or they don't care to know it.
It didn't really do much for me (I was caught on the line between "a lot of this I vaguely remember" and "this part I don't remember, and at this moment, I'm hungry and tired and my brain is resisting the effort ), but I may come back again in a more leisurely moment and see if I can get more out of it.
), but I may come back again in a more leisurely moment and see if I can get more out of it.
Even if I don't, I appreciate what looks to have been a substantial amount of work you've given to the community in drafting and illustrating the post. Sadly, it's probably going to be hard to be sure just how many people read it and how much they get out of it, because some may not comment, so you may not be in a situation of "perfect information" in making your calculus of whether to continue the series, or which direction to take.
Thanks for doing this, and for the earlier sodium post, which I don't think I commented on, but thought was a good contribution to explaining something a lot of people don't seem to understand. The connection to diet and common dieting misconceptions seemed a little more direct in the sodium post, but obviously it's up to you to decide how direct you want that connection to be, and this may be just the information some people want or need.
1 -
lynn_glenmont wrote: »Aaron_K123 wrote: »
Oh yeah feel free to pick on them I'm not shying away from criticism. I just think people might be right that this time I crossed a line into being overly technical.
If I post on a topic not many people know about but is at a level that is engaging and a topic that interests people on the forums then it could be useful and informative for the community. If I post on a topic not many people know about at a level that is not engaging and on something that borders on off-topic then it won't be that useful. Its easy to cross that line if one gets technical unfortunately. You risk going to a level of detail where people reading it either already know it or they don't care to know it.
It didn't really do much for me (I was caught on the line between "a lot of this I vaguely remember" and "this part I don't remember, and at this moment, I'm hungry and tired and my brain is resisting the effort ), but I may come back again in a more leisurely moment and see if I can get more out of it.
), but I may come back again in a more leisurely moment and see if I can get more out of it.
Even if I don't, I appreciate what looks to have been a substantial amount of work you've given to the community in drafting and illustrating the post. Sadly, it's probably going to be hard to be sure just how many people read it and how much they get out of it, because some may not comment, so you may not be in a situation of "perfect information" in making your calculus of whether to continue the series, or which direction to take.
Thanks for doing this, and for the earlier sodium post, which I don't think I commented on, but thought was a good contribution to explaining something a lot of people don't seem to understand. The connection to diet and common dieting misconceptions seemed a little more direct in the sodium post, but obviously it's up to you to decide how direct you want that connection to be, and this may be just the information some people want or need.
Yeah that basically sums up my fear. I'm not afraid of not be liked, I'm not afraid of being criticized. I'm afraid of the possibility I am wasting my time. Not meant as an indictment of my audience, meant as I have chosen to phrase this in such a way that most people will get nothing out of it and therefore the time I spent making the post will not be worth it.1 -
Anyone else feel like they're imprinting on Aaron like a baby duck?5
-
Bumping old threads and this one never got enough attention.3
-
Some of us enjoy reading the "overly technical" stuff. It's a lot more interesting than the ten thousandth "anybody doing keto?", "how often should you weigh", or "how to beat sugar addiction" threads.Aaron_K123 wrote: »
Oh yeah feel free to pick on them I'm not shying away from criticism. I just think people might be right that this time I crossed a line into being overly technical.
If I post on a topic not many people know about but is at a level that is engaging and a topic that interests people on the forums then it could be useful and informative for the community. If I post on a topic not many people know about at a level that is not engaging and on something that borders on off-topic then it won't be that useful. Its easy to cross that line if one gets technical unfortunately. You risk going to a level of detail where people reading it either already know it or they don't care to know it. 2
2 -
diannethegeek wrote: »Bumping old threads and this one never got enough attention.
We need a sticky thread with just links to @Aaron_K123 posts.
I'll go suggest it. Just not sure which forum it would belong in.
Done.
In here
http://community.myfitnesspal.com/en/discussion/10260479/nominate-posts-for-announcement-status-stickies#latest
Now to just get some agreement 2
2 -

Just bumping useful posts. Don't mind me.1
This discussion has been closed.
Categories
- All Categories
- 1.4M Health, Wellness and Goals
- 398.2K Introduce Yourself
- 44.7K Getting Started
- 261K Health and Weight Loss
- 176.4K Food and Nutrition
- 47.7K Recipes
- 233K Fitness and Exercise
- 462 Sleep, Mindfulness and Overall Wellness
- 6.5K Goal: Maintaining Weight
- 8.7K Goal: Gaining Weight and Body Building
- 153.5K Motivation and Support
- 8.4K Challenges
- 1.4K Debate Club
- 96.5K Chit-Chat
- 2.6K Fun and Games
- 4.8K MyFitnessPal Information
- 12 News and Announcements
- 21 MyFitnessPal Academy
- 1.6K Feature Suggestions and Ideas
- 3.2K MyFitnessPal Tech Support Questions