A couple of things I don't understand about weight loss

FrumMama
Posts: 79 Member
1) How does weight actually "get lost." For example, if I weigh myself and I weigh 100 pounds (yeah right). And then I eat a 16 oz steak, a pound of potatoes, and a pound of green beans. I should gain three pounds, right? But there's not necessarily the same amount of calories in each of those, so I'll probably end up gaining more from the steak than the green beans. Why? Isn't the steak still in me?
2) How does weight "get gained"? For example, at night I weigh myself and weigh 100 pounds (yeah right). The next morning, before eating, I weigh myself and weigh 101.5 pounds. How does that happen?? This just happened to me the other day. How does this make sense?
3) What does exercise do? Again, there's this "Law of Conservation of Matter" thing we learned in high school. Stuff doesn't just disappear. So how do we lose weight?
Sorry if these are stupid questions, I just don't get it.
2) How does weight "get gained"? For example, at night I weigh myself and weigh 100 pounds (yeah right). The next morning, before eating, I weigh myself and weigh 101.5 pounds. How does that happen?? This just happened to me the other day. How does this make sense?
3) What does exercise do? Again, there's this "Law of Conservation of Matter" thing we learned in high school. Stuff doesn't just disappear. So how do we lose weight?
Sorry if these are stupid questions, I just don't get it.
3
Replies
-
Your body uses energy aka burns calories keeping our bodies alive. It takes energy to pump that heart, expand those lungs etc. If you eat 3 pounds of food yes immediately you will weigh 3 pounds more but then as body digests food the calorie content of the food will become more relevant.
I cant answer your second question, I dont often weigh myself at night but if I do and then weigh again in the morning I always weigh less (providing of course I havent eaten/drunk anything in between).
Exercise burns more energy, we use a certain amount to live also know as BMR but then it takes energy to actually move that body around. Like a car it takes x amount of fuel to keep a car running while staionary. When you drive you use more fuel, drive faster and you'll use more, pick up a passenger and you'll need more fuel, drop off some luggage and it will use less fuel. Where does all the petrol go? We see some come out the exhaust as smoke..........
4 -
You breath in oxygen. O2. You breath out carbon dioxide. CO2. That carbon atom is where your weight is lost, in that exchange, through respiration.
The answer to "where does a plants mass come from?" Is the same answer in reverse.
Yes I know there is also nitrogen exchange but carbon from respiration is the primary source.
So short answer...where does the weight go? You breath it out.
Fancier answer food is primarily hydrocarbons with some nitrogen. The oxygen and hydrogen that aren't incorporated into your own macromolecules are formed into H20, water, and excreted in urine. Nitrogen waste is converted to uric acid and also goes out as waste. Undigestables go out as solid waste. Carbon waste goes out via your breath.
Fat is pure hydrocarbon, no nitrogen. You burn fat you convert it to water which you excrete and carbon you breath out.72 -
Oh, that is the best answer ever. I've read the same kind of thing lots of times, but the simplicity of this is beautiful.
Thanks AaronK, you are a genius at making science stuff understandable!9 -
pebble4321 wrote: »Oh, that is the best answer ever. I've read the same kind of thing lots of times, but the simplicity of this is beautiful.
Thanks AaronK, you are a genius at making science stuff understandable!
Any elegance is nature herself my friend.9 -
When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.8 -
1) How does weight actually "get lost." For example, if I weigh myself and I weigh 100 pounds (yeah right). And then I eat a 16 oz steak, a pound of potatoes, and a pound of green beans. I should gain three pounds, right? But there's not necessarily the same amount of calories in each of those, so I'll probably end up gaining more from the steak than the green beans. Why? Isn't the steak still in me?
Right after eating them, you would weigh more or less 3 pounds more. Yet, right away your body starts to digest them and extract the nutrients and energy from them. That takes energy as well. You are correct that the amount of energy you would get from eating say the steak compared to the beans would be different because there is more energy stored in the steak than in the beans largely because of the fat content. (4 calories per gram of protein and carbohydrate compared to 9 per gram of fat), but it really comes down to how your body uses that energy. Some will be used to do things like digest your food, keep your heart beating, and the rest. That energy is used and results in CO2 being breathed out, so it doesn't become fat. Some is stored in your liver and muscles as glycogen to be used later as a quickly accessible energy source, and some will be stored as fat. As digestion does its work the matter has energy extracted from it, and it is used in these various ways by the body, and what cannot be digested comes out the other end as waste.2) How does weight "get gained"? For example, at night I weigh myself and weigh 100 pounds (yeah right). The next morning, before eating, I weigh myself and weigh 101.5 pounds. How does that happen?? This just happened to me the other day. How does this make sense?
Weight gets gained for various reasons, but the only one really important is fat storage which happens when the energy (calories) you eat that are extracted by digestion exceed the amount needed to maintain your body and power your daily activity and exercise. Weigh can also go up because of water being retained either because a person ate more sodium than they usually do and the body retains water to deal with it, or they have exercised more intensely or a different exercise program than usual in which case water is used as part of the process of repairing the micro damage to muscles so they become stronger and able to do things better. It also goes up if food is going slower through your digestive tract due to lack of fiber, too little water or fat or maybe even an large meal that was within your calorie goal, but needs longer to digest. That stuff still digesting has weight as well.
So your 100 to 101.5 could be a whole host of things. Assuming no further consumption of food or water, it is sort of confounding, but I personally would think it is just the inaccuracies of the average scale. The scales available at the store are not super accurate and many things can cause small inaccurate readings and 1.5 pounds would be considered that.3) What does exercise do? Again, there's this "Law of Conservation of Matter" thing we learned in high school. Stuff doesn't just disappear. So how do we lose weight?
Exercise takes the energy in your body and turns it into motion. The resulting process that Oxygen in and exhales Carbon Dioxide and that Carbon atom added is primarily where that "mass" goes. It isn't created or destroyed it just changes state in a way that leaves your body.Sorry if these are stupid questions, I just don't get it.
Not stupid at all.
7 -
#2 likely just variations in your scale. Don't worry about that though if you weigh yourself regularly you can still track your weightloss....the error will cancel out over time and the trend will be the same.1
-
You might find this interesting. Kind of "shows" what posters above, especially @Aaron_K123, have been telling.
 https://youtu.be/vuIlsN32WaE 18
https://youtu.be/vuIlsN32WaE 18 -
You might find this interesting. Kind of "shows" what posters above, especially @Aaron_K123, have been telling.
 https://youtu.be/vuIlsN32WaE
https://youtu.be/vuIlsN32WaE
Nice talk, that covers it well. Learned something myself, didn't know the ratio of oxygen that went out as water versus what went out as C02 from hydrocarbons you injest or use from your fat stores. I knew most went out via breath but not what percent.
1 -
Wow, thanks, that makes a lot of sense! Just one more question, for all you science guys out there. Why doesn't weightlifting burn calories? You would think that you're working your muscles, so the same thing should happen...0
-
Weight lifting does burn calories, it's just much harder to accurately predict the calories burned.
Strength training is incredibly variable depending on the muscle groups used, current level of fitness, poundage being lifted, rest periods between sets - the way your body is using the energy is harder to measure than when you're doing an aerobic exercise like running.2 -
You might find this interesting. Kind of "shows" what posters above, especially @Aaron_K123, have been telling.
 https://youtu.be/vuIlsN32WaE
https://youtu.be/vuIlsN32WaE
Mind....blown.
This is going on my FB. 0
0 -
Wow, thanks, that makes a lot of sense! Just one more question, for all you science guys out there. Why doesn't weightlifting burn calories? You would think that you're working your muscles, so the same thing should happen...
It does burn calories but not like steady state cardio. Its not as easily measured due to many factors like weight, intensity, reps, etc.
Sleeping burns calories- so why would you think a lifting routine wouldnt? 0
0 -
-
Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?1 -
Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?
You should watch the last 5ish minutes (really should watch the whole video ^^) He addresses that question.
Actually can FF to 17:38 in the video.1 -
MommyMeggo wrote: »Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?
You should watch the last 5ish minutes (really should watch the whole video ^^) He addresses that question.
Actually can FF to 17:38 in the video.
cool cool, wish there was a bookmark feature on here, YT is blocked at work- kinda their hint that I should be working.0 -
MommyMeggo wrote: »Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?
You should watch the last 5ish minutes (really should watch the whole video ^^) He addresses that question.
Actually can FF to 17:38 in the video.
cool cool, wish there was a bookmark feature on here, YT is blocked at work- kinda their hint that I should be working.
If you mean on MFP, just select the star up at the top and it will be bookmarked.2 -
MommyMeggo wrote: »Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?
You should watch the last 5ish minutes (really should watch the whole video ^^) He addresses that question.
Actually can FF to 17:38 in the video.
cool cool, wish there was a bookmark feature on here, YT is blocked at work- kinda their hint that I should be working.
Oh LOl yes.
Basically what he said is with hyperventilating there is no hormone released (which comes from eating less/moving more ) that breaks apart the bigger fat molecule into 3 smaller ones (triglycerides) which allows the CO2 to escape so it can be released and leave the body.0 -
rileysowner wrote: »MommyMeggo wrote: »Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?
You should watch the last 5ish minutes (really should watch the whole video ^^) He addresses that question.
Actually can FF to 17:38 in the video.
cool cool, wish there was a bookmark feature on here, YT is blocked at work- kinda their hint that I should be working.
If you mean on MFP, just select the star up at the top and it will be bookmarked.
ahh! thanks! I thought that was for following a thread/ getting updates but it's just a bookmark after all.0 -
Aaron_K123 wrote: »When you exercise rate of oxygen/carbon exchange goes up and you breath harder to compensate.
Don't get the impression that hyperventilating will shed pounds though....doesn't work like that.
haha as I was reading that I was thinking "hey GUYS! guys! I think I've stumbled on a-oh."
So briefly, why doesn't hyperventilating work?
Basically weight loss through respiration is exhalation of CO2, not the physical act of exhaling. If you hypervenilate you aren't actually releasing any more CO2 than if you were breathing normally, you are just breathing out basically what you just breathed in.
The only way to accelerate weight loss is to take in less material to begin with (eat less) or to export more material (break down fats through exercise and then exhale the CO2 and pee out the H2O that results). When you exercise you produce more CO2 so your body triggers you to breath harder and more to export it. If you aren't exercising and you just decide to breath hard you aren't actually getting rid of any more CO2...you are just breathing hard for no reason and if anything are just going to make yourself dizzy.
Another fun fact, lack of oxygen isn't actually what triggers the feeling of needing to breath...is CO2 production and build up. That is why if you enter a room that has no oxygen in it but some other gas (like CO2 for example) you won't notice or feel like you are asphixiating...you'll continue to breath so you will continue to release C02 you just won't be getting any oxygen. As a result within a matter of seconds to a few minutes you will just keel over and pass out without ever having noticed you weren't actually breathing oxygen. That is why gases like C02 in a closed space are so dangerous. People assume that if you aren't getting oxygen you'd know because you'd be gasping for air...but that isn't the case.8 -
So, why then do I gain weight (or plateau) if I don't eat enough but still work out hard and eat clean? That really doesn't make a lot of sense to me. If I don't chart, I think I am eating too much... but in reality I am eating way too little.0
-
You might find this interesting. Kind of "shows" what posters above, especially @Aaron_K123, have been telling.
 https://youtu.be/vuIlsN32WaE
https://youtu.be/vuIlsN32WaE
AWESOME! Thanks for sharing!1 -
1) How does weight actually "get lost." For example, if I weigh myself and I weigh 100 pounds (yeah right). And then I eat a 16 oz steak, a pound of potatoes, and a pound of green beans. I should gain three pounds, right? But there's not necessarily the same amount of calories in each of those, so I'll probably end up gaining more from the steak than the green beans. Why? Isn't the steak still in me?
2) How does weight "get gained"? For example, at night I weigh myself and weigh 100 pounds (yeah right). The next morning, before eating, I weigh myself and weigh 101.5 pounds. How does that happen?? This just happened to me the other day. How does this make sense?
3) What does exercise do? Again, there's this "Law of Conservation of Matter" thing we learned in high school. Stuff doesn't just disappear. So how do we lose weight?
Sorry if these are stupid questions, I just don't get it.
The weight you gain right after eating some food has nothing to do with the calories...if you eat a 12 oz steak, you're going to gain roughly 12 Oz...because that's how much the steak weighs...nothing to do with the calories in said steak.
A calorie is a unit of energy...our bodies are super awesome machines that utilize energy (calories) to function. You have a certain level of calories that are required for to fuel everything from merely existing to your day to day to your exercise. When you consume energy at levels commensurate with what your body requires to do all of those things, you maintain...when you consume energy in excess of what your body requires, that energy is stored for later use as body fat...basically your backup generator. When you consume less energy than your body requires to perform all of those functions then your backup generator kicks on and you burn body fat to make up for the energy deficiency. That's a bit oversimplified, but that is the general process.
Exercise increases your body's energy expenditure...so let's say you maintain weight on 2000 calories per day with no exercise...now lets say you add in exercise and you start burning 500 calories per workout...that's 500 units of energy that your body wasn't burning before...so this would move your maintenance level of calories to 2500 if you wanted to maintain the status quo.
In regards to question two, body weight isn't static...your body is made up substantially of water and from day to day, the actual % makeup changes...water has mass and thus weight...not all weight is fat...fluctuations in water composition are going to show up as fluctuations on the scale. Beyond that, you always have varying degrees of waste in your system. Body weight isn't static...it fluctuates constantly...losing or gaining fat isn't about looking at the day to day numbers...it's about watching overall trends.2 -
this is the best thread i have seen in ages, thanks @joinn68 for that video!1
-
So, why then do I gain weight (or plateau) if I don't eat enough but still work out hard and eat clean? That really doesn't make a lot of sense to me. If I don't chart, I think I am eating too much... but in reality I am eating way too little.
Weight isn't just fat, weight is also water. If you burn fat and accumulate water then your weight isn't going to change. Often when you "work out hard" your body retains a lot of water to help with muscle repair. These "plateaus" are usually on the order of one or two weeks long, doesn't actually mean you aren't losing fat.
Eating too little doesn't make you "plateau", that is the often claimed "starvation mode" of weight loss which is a myth.5 -
Also like the video says people have this mistaken concept that somehow fat is literally burned and turned to nothingness and energy...that isn't what happens...actually that isn't even what happens when you literally burn something. The same amount of atoms exist before and after you "burn" fat.
Burning something, ie with fire, oxidizes the molecules. Oxygen combines with bonds and breaks them breaking the molecules up...which on a side note is why fire requires oxygen, becauses its a reaction utilizing oxygen. When you burn hydrocarbons in a fire it produces CO2 and water (which since it is hot comes out as vapor). Fire is just heat and light release from this reaction running full speed, the energy just released instead of converted into something else. In terms of what flames in a fire consist of, its pretty much carbon dioxide and water vapor. Our bodies bascially carry out the exact same reaction but in a much more controlled manner that harnesses the energy using it to create other bonds rather than releasing it as light and heat. Similar in a way to the difference between a nuclear reactor (controlled reaction harnessing the energy to turn a turbine) and a nuclear bomb (run-away reaction just releasing the energy as heat and sound). So when we say we "burn" fat in a way that is literal and accurate but in another way it isn't because when people think of either they picture matter being converted into energy which isn't what is happening. Neither fire nor our metabolism actually destroys any matter, it just coverts it to other forms while releasing the energy in the bonds that were holding those atoms together.
4 -
Plants run the exact same metabolic reaction we do, just in reverse.
So take something like sugar. Sugar, like fat, is just a form of hydrocarbon. To qualify as a sugar you just need the molecular formula CxH2xOx. So glucose for example is C6H12O6.
Heterotrophs, like animals, are living beings that use organic molecules for chemical energy. They break down organic molecules like hydrocarbons for energy by oxidation utilizing oxygen . So C6H12O6 plus 6 O2 becomes 6H2O + 6CO2. This by the way is why we need to breath in oxygen and why we exhale carbon dioxide.
Autotrophs, like plants, are living beings that use the sun (or inorganic compounds) for energy to produce organic molecules basically they run that reaction in the opposite direction. So 6H2O + 6CO2 becomes C6H12O6 plus 6 O2. This is why plants need to "breath" in carbon dioxide and then "exhale" oxygen.
Almost all of our bodies metabolic functions and macromolecules are built and function off this interplay between oxygen, carbon dioxide and water.
You probably hear sugars refered to as carbohydrates...that is because they are all carbon that has been hydrated...some form of CH2O. That goes for sugars and starches anything from glucose to cellulose to amylopectin. Not all carbohydrates are sweet, not all carbohydrates are even digestable by us (think grass)...but they all share the same basic forumula and they are all metabolized in a similar way as an interchange between carbon dioxide water and oxygen.1 -
This is basically the building blocks of fat...
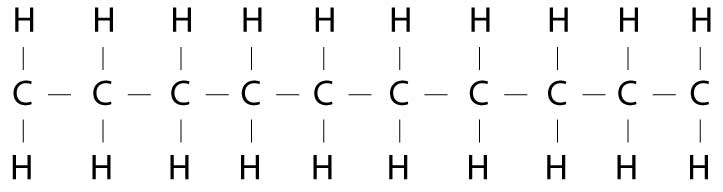
Carbon and hydrogen (toss in a few oxygens and other stuff but 99% carbon and oxygen)
As has been mentioned the carbon, once broken down goes out as CO2. And the hydrogen is also mixed with oxygen to make water. Excreted or used in other processes. Well, partially true.
Those carbons are also used to make cholesterol (all your cells have walls from fat) and every hormone and signaling molecule and glucose molecule, etc, etc, made by your liver comes mostly from fat stores. No fat... no life.
0 -
Aaron_K123 wrote: »pebble4321 wrote: »Oh, that is the best answer ever. I've read the same kind of thing lots of times, but the simplicity of this is beautiful.
Thanks AaronK, you are a genius at making science stuff understandable!
Any elegance is nature herself my friend.
Nope, not just nature. I've tried to explain that to folks myself, and didn't come close to your simplicity/clarity. Good Englishing, dude! 1
1
This discussion has been closed.
Categories
- All Categories
- 1.4M Health, Wellness and Goals
- 398.4K Introduce Yourself
- 44.7K Getting Started
- 261K Health and Weight Loss
- 176.4K Food and Nutrition
- 47.7K Recipes
- 233K Fitness and Exercise
- 462 Sleep, Mindfulness and Overall Wellness
- 6.5K Goal: Maintaining Weight
- 8.7K Goal: Gaining Weight and Body Building
- 153.5K Motivation and Support
- 8.4K Challenges
- 1.4K Debate Club
- 96.5K Chit-Chat
- 2.6K Fun and Games
- 4.7K MyFitnessPal Information
- 17 News and Announcements
- 21 MyFitnessPal Academy
- 1.5K Feature Suggestions and Ideas
- 3.2K MyFitnessPal Tech Support Questions