Biochemistry answers to weight loss questions: Where does the weight go?

Aaron_K123
Posts: 7,122 Member
I see this question on the thread a lot:
"Okay I get that if I eat less calories than I use I will lose weight, but where does that weight actually go?"
The assumption seems to be that you either poop it out somehow or that you literally convert mass into energy like you were some sort of nuclear reactor. Neither one of these is correct. The answer comes from biochemistry and I thought I'd try to give a layman explanation of what is going on when you eat food and do work. By explaining that it becomes evident where the weight goes.
First lets address the idea that you lose weight by essentially pooping it out. When you eat food enters your mouth and goes through your gastrointestinal tract. That is basically a tube from your mouth to your *kitten* that, if you think about it, is actually "outside" of your body. The parts you can digest are broken down into smaller soluble pieces and transferred through your intestinal wall to your blood. What remains behind just passes through you and as such was never really inside you in the first place. Nothing substantial crosses that lining in the opposite direction so what you poop out is just what you couldn't digest mixed with the bacteria that are in your gut. The weight you lose from pooping is just whatever you ate that you couldn't digest mixed with bacteria that is in your gut, it is not weight that was ever a part of you. Therefore you do not lose weight from your body through excrement.
The next idea is that you convert fat into energy and fat has mass and energy doesn't so you are literally just destroying mass inside of you. The more fat you convert the more weight you lose as a result. That doesn't happen. We do breakdown fats and other biomolecules to get energy but the mass is conserved, you are just breaking it down into parts that weigh the same amount as the whole. The only thing that converts mass to energy is a nuclear reaction and our bodies aren't nuclear reactors. Our body temperature is about 37 degrees celsius, not the 100 million degrees nuclear fusion would require. Therefore you don't lose weight from conversion of mass to energy.
So how do we lose weight? Well to understand that we have to look at how we do convert the food we eat into usable energy. The food we eat has one thing in common, the part that gives us sustenance in the form of calories are molecules comprised of carbon (C), hydrogen (H) and oxygen (O). Fats, or hydrocarbons, are made of just C and H. Protein is made from units called amino acids that are composed of carbon, hydrogen, oxygen and nitrogen. Carbohydrates are carbon, C, combined with water, H20, to form a carbo-hydrate CxH2x0x. Glucose for example is C6H12O6 which is essentially 6 carbons and 6 waters. Molecules that are based around carbon as these are are referred to as organic molecules.
When you eat pasta your GI tract breaks down the starches into their constitutive soluble parts which are glucose molecules. Those molecules then transfer across your intestinal lining to your blood raising your blood sugar which triggers insulin which triggers your cells to uptake glucose. Glucose is then broken down by a long series of enzymatic reactions which are oxidative, they use oxygen to break bonds in the molecule. When a chemical bond is broken some energy is released and these enzymes capture that release of energy by forming of a new bond in a "currency" molecule converting adenosine diphosphate into adenosine triphosphate (ATP). ATP is used almost like a battery for universal energy storage. Other processes in our body can be powered by these batteries which allows the production of ATP to essentially power our bodies by breaking the bond in ATP to form ADP which can then be converted back to ATP by the breakdown of bonds in food molecules.
Most of that energy capture and coupling happens through the electron transport chain (ETC) which is too complex to cover in a thread and would confuse/bore the hell out of people as I spent pages and pages trying to explain. I guess the analogy for the ETC would be picture a society where energy from lots of different specialized sources is used to transport water uphill and when that water flows back down through a channel it flows through a water-wheel that turns a turbine that produces energy that is put into rechargable batteries that are usable by everyone. In this analogy the water is protons and the energy input is food while the rechargable batteries are ATP molecules.
For those who are curious here is the ETC "water wheel" ATP synthase in action.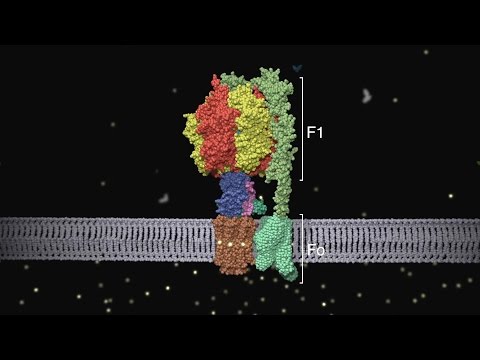 https://www.youtube.com/watch?v=b_cp8MsnZFA
https://www.youtube.com/watch?v=b_cp8MsnZFA
So what does that end up looking like in total? What happens to that glucose molecule?
Well glucose, C6H12O6 is broken apart by oxygen, O2, to smaller water and carbon dioxide molecules with the released energy captured in ATP molecules with some waste energy released as heat.
C6H12O6 + 6 O2 + 32 ADP >>>> 6 C02 + 6 H20 + 32 ATP + heat
So when we say that we "burn" calories it is just a phrase but it is a surprisingly accurate one. Burning something is also an oxidation reaction, which is why fire requires oxygen. If you literally burn glucose by setting it on fire then the reaction that is occuring is:
C6H12O6 + 6 O2 >>>> 6 C02 + 6 H20 + heat.
The only difference between this and what happens in your body is that in your body the reaction is carried out by enzymes that capture most of that energy in the form of bonds in ATP while in a fire that energy is entirely converted into heat.
So lets track the mass here. ADP >>> ATP is cyclical, the ADP and ATP stay in your body its just a way of storing energy so that isn't it. Water is kept in balance in your body, sure you excrete water in the form of urine, sweat, tears, etc but you also need to drink water to keep hydrated and keep your water levels the same so that isn't it. You breath in oxygen to carry out these oxidative reactions and you breath out carbon dioxide as a waste product. That isn't balanced, carbon dioxide is essentially oxygen plus carbon. So if you breath in 6 oxygen molecules and breath out 6 carbon dioxide molecules you have essentially lost 6 carbons from your body.
Now if all you do is burn the glucose you eat for energy then that carbon comes from the glucose you ate so you would not lose any weight (the C you breath out is balanced by the C you just ate). However if you take in less calories than you need to power your body you will break down glycogen (stored glucose) and/or fats (stored hydrocarbons) within your body by oxidizing them from breathed in oxygen and then breath out carbon dioxide. As a result, you lose weight. If, however, you eat more calorically than your body requires for energy your body will store the excess in the form of either glycogen (for glucose) or triglycerides (for fats) and since those molecules have weight you will gain weight. Although I used glucose as the example here the sample principle would hold true of fats as well. Proteins also contain nitrogen and waste nitrogen is excreted in the form of uric acid in our urine.
So what is the answer? When you lose weight the weight you are losing is being breathed out in the form of carbon in carbon dioxide. The amount of carbon dioxide you breath out is connected to how metabolically active you are which in turn is connected to how physically active you are being. If you go for a run you will breakdown molecules to create energy which will require more oxygen and expel more carbon dioxide which is why you have to breath harder. If, instead, you just sit on your couch and breath hard you are taking more breaths but you aren't actually producing more carbon dioxide all you are doing is expelling less carbon dioxide per exhale...that is just hyperventilating and won't do anything other than making you a bit light headed.
"Okay I get that if I eat less calories than I use I will lose weight, but where does that weight actually go?"
The assumption seems to be that you either poop it out somehow or that you literally convert mass into energy like you were some sort of nuclear reactor. Neither one of these is correct. The answer comes from biochemistry and I thought I'd try to give a layman explanation of what is going on when you eat food and do work. By explaining that it becomes evident where the weight goes.
First lets address the idea that you lose weight by essentially pooping it out. When you eat food enters your mouth and goes through your gastrointestinal tract. That is basically a tube from your mouth to your *kitten* that, if you think about it, is actually "outside" of your body. The parts you can digest are broken down into smaller soluble pieces and transferred through your intestinal wall to your blood. What remains behind just passes through you and as such was never really inside you in the first place. Nothing substantial crosses that lining in the opposite direction so what you poop out is just what you couldn't digest mixed with the bacteria that are in your gut. The weight you lose from pooping is just whatever you ate that you couldn't digest mixed with bacteria that is in your gut, it is not weight that was ever a part of you. Therefore you do not lose weight from your body through excrement.
The next idea is that you convert fat into energy and fat has mass and energy doesn't so you are literally just destroying mass inside of you. The more fat you convert the more weight you lose as a result. That doesn't happen. We do breakdown fats and other biomolecules to get energy but the mass is conserved, you are just breaking it down into parts that weigh the same amount as the whole. The only thing that converts mass to energy is a nuclear reaction and our bodies aren't nuclear reactors. Our body temperature is about 37 degrees celsius, not the 100 million degrees nuclear fusion would require. Therefore you don't lose weight from conversion of mass to energy.
So how do we lose weight? Well to understand that we have to look at how we do convert the food we eat into usable energy. The food we eat has one thing in common, the part that gives us sustenance in the form of calories are molecules comprised of carbon (C), hydrogen (H) and oxygen (O). Fats, or hydrocarbons, are made of just C and H. Protein is made from units called amino acids that are composed of carbon, hydrogen, oxygen and nitrogen. Carbohydrates are carbon, C, combined with water, H20, to form a carbo-hydrate CxH2x0x. Glucose for example is C6H12O6 which is essentially 6 carbons and 6 waters. Molecules that are based around carbon as these are are referred to as organic molecules.
When you eat pasta your GI tract breaks down the starches into their constitutive soluble parts which are glucose molecules. Those molecules then transfer across your intestinal lining to your blood raising your blood sugar which triggers insulin which triggers your cells to uptake glucose. Glucose is then broken down by a long series of enzymatic reactions which are oxidative, they use oxygen to break bonds in the molecule. When a chemical bond is broken some energy is released and these enzymes capture that release of energy by forming of a new bond in a "currency" molecule converting adenosine diphosphate into adenosine triphosphate (ATP). ATP is used almost like a battery for universal energy storage. Other processes in our body can be powered by these batteries which allows the production of ATP to essentially power our bodies by breaking the bond in ATP to form ADP which can then be converted back to ATP by the breakdown of bonds in food molecules.
Most of that energy capture and coupling happens through the electron transport chain (ETC) which is too complex to cover in a thread and would confuse/bore the hell out of people as I spent pages and pages trying to explain. I guess the analogy for the ETC would be picture a society where energy from lots of different specialized sources is used to transport water uphill and when that water flows back down through a channel it flows through a water-wheel that turns a turbine that produces energy that is put into rechargable batteries that are usable by everyone. In this analogy the water is protons and the energy input is food while the rechargable batteries are ATP molecules.
For those who are curious here is the ETC "water wheel" ATP synthase in action.
 https://www.youtube.com/watch?v=b_cp8MsnZFA
https://www.youtube.com/watch?v=b_cp8MsnZFASo what does that end up looking like in total? What happens to that glucose molecule?
Well glucose, C6H12O6 is broken apart by oxygen, O2, to smaller water and carbon dioxide molecules with the released energy captured in ATP molecules with some waste energy released as heat.
C6H12O6 + 6 O2 + 32 ADP >>>> 6 C02 + 6 H20 + 32 ATP + heat
So when we say that we "burn" calories it is just a phrase but it is a surprisingly accurate one. Burning something is also an oxidation reaction, which is why fire requires oxygen. If you literally burn glucose by setting it on fire then the reaction that is occuring is:
C6H12O6 + 6 O2 >>>> 6 C02 + 6 H20 + heat.
The only difference between this and what happens in your body is that in your body the reaction is carried out by enzymes that capture most of that energy in the form of bonds in ATP while in a fire that energy is entirely converted into heat.
So lets track the mass here. ADP >>> ATP is cyclical, the ADP and ATP stay in your body its just a way of storing energy so that isn't it. Water is kept in balance in your body, sure you excrete water in the form of urine, sweat, tears, etc but you also need to drink water to keep hydrated and keep your water levels the same so that isn't it. You breath in oxygen to carry out these oxidative reactions and you breath out carbon dioxide as a waste product. That isn't balanced, carbon dioxide is essentially oxygen plus carbon. So if you breath in 6 oxygen molecules and breath out 6 carbon dioxide molecules you have essentially lost 6 carbons from your body.
Now if all you do is burn the glucose you eat for energy then that carbon comes from the glucose you ate so you would not lose any weight (the C you breath out is balanced by the C you just ate). However if you take in less calories than you need to power your body you will break down glycogen (stored glucose) and/or fats (stored hydrocarbons) within your body by oxidizing them from breathed in oxygen and then breath out carbon dioxide. As a result, you lose weight. If, however, you eat more calorically than your body requires for energy your body will store the excess in the form of either glycogen (for glucose) or triglycerides (for fats) and since those molecules have weight you will gain weight. Although I used glucose as the example here the sample principle would hold true of fats as well. Proteins also contain nitrogen and waste nitrogen is excreted in the form of uric acid in our urine.
So what is the answer? When you lose weight the weight you are losing is being breathed out in the form of carbon in carbon dioxide. The amount of carbon dioxide you breath out is connected to how metabolically active you are which in turn is connected to how physically active you are being. If you go for a run you will breakdown molecules to create energy which will require more oxygen and expel more carbon dioxide which is why you have to breath harder. If, instead, you just sit on your couch and breath hard you are taking more breaths but you aren't actually producing more carbon dioxide all you are doing is expelling less carbon dioxide per exhale...that is just hyperventilating and won't do anything other than making you a bit light headed.
Tagged:
87
Replies
-
Thank you for taking the time to post threads like this, Aaron. Very interesting and educational.3
-
Very insightful, thanks for the information!!0
-
So if I'm breathing out my weight that I'm losing, does that mean my kids are breathing me in as they're growing?
 11
11 -
This just reaffirms my crush on Aaron. Another great post.7
-
Dude, thank you for this. It's refreshing to see biochemistry, as much as others get turned off by it, I appreciate the hell out of it.4
-
Aaron--love your explanations. Thanks for taking the time to educate. I can say I learned something today.2
-
Thank you!!!! This is very interesting. I know this has been highly requested!0
-
lightenup2016 wrote: »So if I'm breathing out my weight that I'm losing, does that mean my kids are breathing me in as they're growing?

Kids no, houseplants yes.
Plants run this reaction in the other direction. Plants take in and combine CO2 with H20 using energy from the sun to form glucose which they keep and oxygen which they expel. Animals take in oxygen and food to break down molecules like glucose to produce energy as well as form H20 and CO2 which they expel.
Therefore a plants mass comes from the carbon from CO2, and if you are expelling that CO2 then yes, they would be incorporating "your" carbon into their "bodies". Only way carbon that is in you ends up in your kid is if you breath out around a fruit tree and later your kids eat the fruit. Circle of life and all.24 -
Nuclear fusion in the Sun occurs at 15.7 million kelvin, which is pretty darn close to 15.7 million F, which is still not going to happen inside a human body. Giggity.
I also want to point out that when anything passes from inside the body to outside by reversing the normal flow across the lining of the intestinal tract, you have a much bigger problem than simply worrying about calorie budgets and energy balance. That big problem includes symptoms of diarrhea, and goes by names such as ebola and marburg.7 -
So If I am understanding what I read correctly, when the body runs out of glycogen stores from food, it breaks down fat stores if you are running in a deficit. The same would apply for dietary fats. The body will burn that off first, then turn to your fat stores. So therefor, weather you are consuming a diet high in carbohysdrates, or a diet high in fat and protiens, your body will burn it off the same no matter what you are eating. I'm so using this the next time my friends try telling me keto really burns off more fat then a conventional diet of simply cutting calories. (Not that I'm dissing those who are doing keto and loving it.)11
-
"Layman's terms"... Didn't realize that equated to maths and formulas! Haha!!!7
-
Doesn't some of it get expelled as water? I thought the breakdown was 86% CO2 and 14% H2O, some of which would be expelled.
I'm thinking as my weight drops, I'm carrying less water than before.0 -
Is there a Biochemistry answers to weight loss questions for dummies thread somewhere???
I'm kidding. Kind of. Good posts though, thank you.4 -
Aaron_K123 wrote: »lightenup2016 wrote: »So if I'm breathing out my weight that I'm losing, does that mean my kids are breathing me in as they're growing?

Kids no, houseplants yes.
Plants run this reaction in the other direction. Plants take in and combine CO2 with H20 using energy from the sun to form glucose which they keep and oxygen which they expel. Animals take in oxygen and food to break down molecules like glucose to produce energy as well as form H20 and CO2 which they expel.
Therefore a plants mass comes from the carbon from CO2, and if you are expelling that CO2 then yes, they would be incorporating "your" carbon into their "bodies". Only way carbon that is in you ends up in your kid is if you breath out around a fruit tree and later your kids eat the fruit. Circle of life and all.
Right, I realized this after I posted it. Still funny, though, to think that my family is breathing in my breath that contains the carbon dioxide from the pounds I lost. We only have one house plant, but it doesn't seem to be benefiting much!2 -
khaleesikhaleesi wrote: »"Layman's terms"... Didn't realize that equated to maths and formulas! Haha!!!
Well, the non-layman explanation is several years of course-work and some rather dense textbooks and that is just to understand the very basic framework of it. If it fits in a forum post you can read in 5 minutes I think it qualifies as layman.
I mean this is what we understand so far about the biochemistry our bodies carry out if you want to get a concept of the non-layman version:
http://biochemical-pathways.com/#/map/1
My example of glucose metabolism starts in quadrant C6 following it to acetyl-CoA and entry into citric acid cycle in F59 -
lightenup2016 wrote: »So if I'm breathing out my weight that I'm losing, does that mean my kids are breathing me in as they're growing?

My kids grow like houseplants......hmmmmm....:)0 -
Aaron_K123 wrote: »I see this question on the thread a lot:
"Okay I get that if I eat less calories than I use I will lose weight, but where does that weight actually go?"
The assumption seems to be that you either poop it out somehow or that you literally convert mass into energy like you were some sort of nuclear reactor. Neither one of these is correct. The answer comes from biochemistry and I thought I'd try to give a layman explanation of what is going on when you eat food and do work. By explaining that it becomes evident where the weight goes.
First lets address the idea that you lose weight by essentially pooping it out. When you eat food enters your mouth and goes through your gastrointestinal tract. That is basically a tube from your mouth to your *kitten* that, if you think about it, is actually "outside" of your body. The parts you can digest are broken down into smaller soluble pieces and transferred through your intestinal wall to your blood. What remains behind just passes through you and as such was never really inside you in the first place. Nothing substantial crosses that lining in the opposite direction so what you poop out is just what you couldn't digest mixed with the bacteria that are in your gut. The weight you lose from pooping is just whatever you ate that you couldn't digest mixed with bacteria that is in your gut, it is not weight that was ever a part of you. Therefore you do not lose weight from your body through excrement.
The next idea is that you convert fat into energy and fat has mass and energy doesn't so you are literally just destroying mass inside of you. The more fat you convert the more weight you lose as a result. That doesn't happen. We do breakdown fats and other biomolecules to get energy but the mass is conserved, you are just breaking it down into parts that weigh the same amount as the whole. The only thing that converts mass to energy is a nuclear reaction and our bodies aren't nuclear reactors. Our body temperature is about 37 degrees celsius, not the 100 million degrees nuclear fusion would require. Therefore you don't lose weight from conversion of mass to energy.
So how do we lose weight? Well to understand that we have to look at how we do convert the food we eat into usable energy. The food we eat has one thing in common, the part that gives us sustenance in the form of calories are molecules comprised of carbon (C), hydrogen (H) and oxygen (O). Fats, or hydrocarbons, are made of just C and H. Protein is made from units called amino acids that are composed of carbon, hydrogen, oxygen and nitrogen. Carbohydrates are carbon, C, combined with water, H20, to form a carbo-hydrate CxH2x0x. Glucose for example is C6H12O6 which is essentially 6 carbons and 6 waters. Molecules that are based around carbon as these are are referred to as organic molecules.
When you eat pasta your GI tract breaks down the starches into their constitutive soluble parts which are glucose molecules. Those molecules then transfer across your intestinal lining to your blood raising your blood sugar which triggers insulin which triggers your cells to uptake glucose. Glucose is then broken down by a long series of enzymatic reactions which are oxidative, they use oxygen to break bonds in the molecule. When a chemical bond is broken some energy is released and these enzymes capture that release of energy by forming of a new bond in a "currency" molecule converting adenosine diphosphate into adenosine triphosphate (ATP). ATP is used almost like a battery for universal energy storage. Other processes in our body can be powered by these batteries which allows the production of ATP to essentially power our bodies by breaking the bond in ATP to form ADP which can then be converted back to ATP by the breakdown of bonds in food molecules.
Most of that energy capture and coupling happens through the electron transport chain (ETC) which is too complex to cover in a thread and would confuse/bore the hell out of people as I spent pages and pages trying to explain. I guess the analogy for the ETC would be picture a society where energy from lots of different specialized sources is used to transport water uphill and when that water flows back down through a channel it flows through a water-wheel that turns a turbine that produces energy that is put into rechargable batteries that are usable by everyone. In this analogy the water is protons and the energy input is food while the rechargable batteries are ATP molecules.
For those who are curious here is the ETC "water wheel" ATP synthase in action. https://www.youtube.com/watch?v=b_cp8MsnZFA
https://www.youtube.com/watch?v=b_cp8MsnZFA
So what does that end up looking like in total? What happens to that glucose molecule?
Well glucose, C6H12O6 is broken apart by oxygen, O2, to smaller water and carbon dioxide molecules with the released energy captured in ATP molecules with some waste energy released as heat.
C6H12O6 + 6 O2 + 32 ADP >>>> 6 C02 + 6 H20 + 32 ATP + heat
So when we say that we "burn" calories it is just a phrase but it is a surprisingly accurate one. Burning something is also an oxidation reaction, which is why fire requires oxygen. If you literally burn glucose by setting it on fire then the reaction that is occuring is:
C6H12O6 + 6 O2 >>>> 6 C02 + 6 H20 + heat.
The only difference between this and what happens in your body is that in your body the reaction is carried out by enzymes that capture most of that energy in the form of bonds in ATP while in a fire that energy is entirely converted into heat.
So lets track the mass here. ADP >>> ATP is cyclical, the ADP and ATP stay in your body its just a way of storing energy so that isn't it. Water is kept in balance in your body, sure you excrete water in the form of urine, sweat, tears, etc but you also need to drink water to keep hydrated and keep your water levels the same so that isn't it. You breath in oxygen to carry out these oxidative reactions and you breath out carbon dioxide as a waste product. That isn't balanced, carbon dioxide is essentially oxygen plus carbon. So if you breath in 6 oxygen molecules and breath out 6 carbon dioxide molecules you have essentially lost 6 carbons from your body.
Now if all you do is burn the glucose you eat for energy then that carbon comes from the glucose you ate so you would not lose any weight (the C you breath out is balanced by the C you just ate). However if you take in less calories than you need to power your body you will break down glycogen (stored glucose) and/or fats (stored hydrocarbons) within your body by oxidizing them from breathed in oxygen and then breath out carbon dioxide. As a result, you lose weight. If, however, you eat more calorically than your body requires for energy your body will store the excess in the form of either glycogen (for glucose) or triglycerides (for fats) and since those molecules have weight you will gain weight. Although I used glucose as the example here the sample principle would hold true of fats as well. Proteins also contain nitrogen and waste nitrogen is excreted in the form of uric acid in our urine.
So what is the answer? When you lose weight the weight you are losing is being breathed out in the form of carbon in carbon dioxide. The amount of carbon dioxide you breath out is connected to how metabolically active you are which in turn is connected to how physically active you are being. If you go for a run you will breakdown molecules to create energy which will require more oxygen and expel more carbon dioxide which is why you have to breath harder. If, instead, you just sit on your couch and breath hard you are taking more breaths but you aren't actually producing more carbon dioxide all you are doing is expelling less carbon dioxide per exhale...that is just hyperventilating and won't do anything other than making you a bit light headed.
You could write a biochemistry textbook. Wow!0 -
You need more paragraphs though. Lol4
-
TL;DR: you breathe it out!
That's SO badass.5 -
I do like how he included the caveat about not just sitting on the couch breathing harder. There goes my weight-loss plan for this evening.
27 -
Crafty_camper123 wrote: »So If I am understanding what I read correctly, when the body runs out of glycogen stores from food, it breaks down fat stores if you are running in a deficit. The same would apply for dietary fats. The body will burn that off first, then turn to your fat stores. So therefor, weather you are consuming a diet high in carbohysdrates, or a diet high in fat and protiens, your body will burn it off the same no matter what you are eating. I'm so using this the next time my friends try telling me keto really burns off more fat then a conventional diet of simply cutting calories. (Not that I'm dissing those who are doing keto and loving it.)
basically yes.
Your body is merely doing what would have occurred anyway, but since eating less than maintenance, back to the fat burning sooner.
Except - insulin also send glucose off to liver and muscle stores, and protein off for usage by everything that needs amino acids.
Typically in diet glucose stores don't get topped off as much as in maintenance mode, so storage and some burning of it for immediate needs gets blood sugar back to normal, insulin drops - back to normal fat burning mode to whatever degree your level of activity requires. All that faster than when eating at maintenance.
Your keto diet expends a few more calories for body to use inefficient process for ketones instead of glucose for brain, and any extra protein converted to glucose for storage.3 -
Ok... I get that 02 =/= CO2 in terms of weight. So some is leaving <bye bye>!!!
How come ATP = ADP? Or are we just rounding there? 0
0 -
Love these threads!1
-
Another great thread. Thank you, Aaron.1
-
Crafty_camper123 wrote: »So If I am understanding what I read correctly, when the body runs out of glycogen stores from food, it breaks down fat stores if you are running in a deficit. The same would apply for dietary fats. The body will burn that off first, then turn to your fat stores. So therefor, weather you are consuming a diet high in carbohysdrates, or a diet high in fat and protiens, your body will burn it off the same no matter what you are eating. I'm so using this the next time my friends try telling me keto really burns off more fat then a conventional diet of simply cutting calories. (Not that I'm dissing those who are doing keto and loving it.)
basically yes.
Your body is merely doing what would have occurred anyway, but since eating less than maintenance, back to the fat burning sooner.
Except - insulin also send glucose off to liver and muscle stores, and protein off for usage by everything that needs amino acids.
Typically in diet glucose stores don't get topped off as much as in maintenance mode, so storage and some burning of it for immediate needs gets blood sugar back to normal, insulin drops - back to normal fat burning mode to whatever degree your level of activity requires. All that faster than when eating at maintenance.
Your keto diet expends a few more calories for body to use inefficient process for ketones instead of glucose for brain, and any extra protein converted to glucose for storage.
Interesting. Thanks for the insight!0 -
Ok... I get that 02 =/= CO2 in terms of weight. So some is leaving <bye bye>!!!
How come ATP = ADP? Or are we just rounding there?
A phosphate group gets used up during the process.
https://www.ncbi.nlm.nih.gov/books/NBK22581/1 -
Crafty_camper123 wrote: »So If I am understanding what I read correctly, when the body runs out of glycogen stores from food, it breaks down fat stores if you are running in a deficit. The same would apply for dietary fats. The body will burn that off first, then turn to your fat stores. So therefor, weather you are consuming a diet high in carbohysdrates, or a diet high in fat and protiens, your body will burn it off the same no matter what you are eating. I'm so using this the next time my friends try telling me keto really burns off more fat then a conventional diet of simply cutting calories. (Not that I'm dissing those who are doing keto and loving it.)
Eh not quite. Before I give my opinion here let me state I am not a physiologist, my expertise is in molecular biology and microbiology not macro animal physiology so don't take my word as total gospel here and feel free others to correct me if I am wrong.
The trigger for metabolizing or storing glucose from glycogen is driven by a homeostatic process regulated by the hormones insulin and glucagon. To say it as simply as possible your pancreas monitors your blood sugar and keeps it level. If it drops too low it produces glucagon which triggers cells that store glycogen to start metabolizing it and releasing glucose into the blood. If your blood sugar is too high it triggers production of insulin which instructs your cells to take up glucose from the blood and either metabolize it for energy or store it as glycogen.
You could technically have high blood sugar but be in caloric deficit (think high sugar diet but low cal) in which case you wouldn't be triggered to break down glycogen while you were metabolizing either fat or the glucose taken up from your blood. Alternatively you could have low blood sugar while being in caloric excess (think high fat low carb diet) in which case you might still break down glycogen to free glucose into your blood while storing fat.
I believe fat is basically long term storage while glycogen is basically quick energy for muscles and bursts of activity.8 -
ButterballBookworm wrote: »I do like how he included the caveat about not just sitting on the couch breathing harder. There goes my weight-loss plan for this evening.
It is kind of like putting the "WARNING: HOT" on coffee cups, just protecting my a** with a disclaimer so I don't end up with dead hyperventilators on my hands.9 -
Aaron_K123 wrote: »I see this question on the thread a lot:
"Okay I get that if I eat less calories than I use I will lose weight, but where does that weight actually go?"
The assumption seems to be that you either poop it out somehow or that you literally convert mass into energy like you were some sort of nuclear reactor. Neither one of these is correct. The answer comes from biochemistry and I thought I'd try to give a layman explanation of what is going on when you eat food and do work. By explaining that it becomes evident where the weight goes.
First lets address the idea that you lose weight by essentially pooping it out. When you eat food enters your mouth and goes through your gastrointestinal tract. That is basically a tube from your mouth to your *kitten* that, if you think about it, is actually "outside" of your body. The parts you can digest are broken down into smaller soluble pieces and transferred through your intestinal wall to your blood. What remains behind just passes through you and as such was never really inside you in the first place. Nothing substantial crosses that lining in the opposite direction so what you poop out is just what you couldn't digest mixed with the bacteria that are in your gut. The weight you lose from pooping is just whatever you ate that you couldn't digest mixed with bacteria that is in your gut, it is not weight that was ever a part of you. Therefore you do not lose weight from your body through excrement.
The next idea is that you convert fat into energy and fat has mass and energy doesn't so you are literally just destroying mass inside of you. The more fat you convert the more weight you lose as a result. That doesn't happen. We do breakdown fats and other biomolecules to get energy but the mass is conserved, you are just breaking it down into parts that weigh the same amount as the whole. The only thing that converts mass to energy is a nuclear reaction and our bodies aren't nuclear reactors. Our body temperature is about 37 degrees celsius, not the 100 million degrees nuclear fusion would require. Therefore you don't lose weight from conversion of mass to energy.
So how do we lose weight? Well to understand that we have to look at how we do convert the food we eat into usable energy. The food we eat has one thing in common, the part that gives us sustenance in the form of calories are molecules comprised of carbon (C), hydrogen (H) and oxygen (O). Fats, or hydrocarbons, are made of just C and H. Protein is made from units called amino acids that are composed of carbon, hydrogen, oxygen and nitrogen. Carbohydrates are carbon, C, combined with water, H20, to form a carbo-hydrate CxH2x0x. Glucose for example is C6H12O6 which is essentially 6 carbons and 6 waters. Molecules that are based around carbon as these are are referred to as organic molecules.
When you eat pasta your GI tract breaks down the starches into their constitutive soluble parts which are glucose molecules. Those molecules then transfer across your intestinal lining to your blood raising your blood sugar which triggers insulin which triggers your cells to uptake glucose. Glucose is then broken down by a long series of enzymatic reactions which are oxidative, they use oxygen to break bonds in the molecule. When a chemical bond is broken some energy is released and these enzymes capture that release of energy by forming of a new bond in a "currency" molecule converting adenosine diphosphate into adenosine triphosphate (ATP). ATP is used almost like a battery for universal energy storage. Other processes in our body can be powered by these batteries which allows the production of ATP to essentially power our bodies by breaking the bond in ATP to form ADP which can then be converted back to ATP by the breakdown of bonds in food molecules.
Most of that energy capture and coupling happens through the electron transport chain (ETC) which is too complex to cover in a thread and would confuse/bore the hell out of people as I spent pages and pages trying to explain. I guess the analogy for the ETC would be picture a society where energy from lots of different specialized sources is used to transport water uphill and when that water flows back down through a channel it flows through a water-wheel that turns a turbine that produces energy that is put into rechargable batteries that are usable by everyone. In this analogy the water is protons and the energy input is food while the rechargable batteries are ATP molecules.
For those who are curious here is the ETC "water wheel" ATP synthase in action. https://www.youtube.com/watch?v=b_cp8MsnZFA
https://www.youtube.com/watch?v=b_cp8MsnZFA
So what does that end up looking like in total? What happens to that glucose molecule?
Well glucose, C6H12O6 is broken apart by oxygen, O2, to smaller water and carbon dioxide molecules with the released energy captured in ATP molecules with some waste energy released as heat.
C6H12O6 + 6 O2 + 32 ADP >>>> 6 C02 + 6 H20 + 32 ATP + heat
So when we say that we "burn" calories it is just a phrase but it is a surprisingly accurate one. Burning something is also an oxidation reaction, which is why fire requires oxygen. If you literally burn glucose by setting it on fire then the reaction that is occuring is:
C6H12O6 + 6 O2 >>>> 6 C02 + 6 H20 + heat.
The only difference between this and what happens in your body is that in your body the reaction is carried out by enzymes that capture most of that energy in the form of bonds in ATP while in a fire that energy is entirely converted into heat.
So lets track the mass here. ADP >>> ATP is cyclical, the ADP and ATP stay in your body its just a way of storing energy so that isn't it. Water is kept in balance in your body, sure you excrete water in the form of urine, sweat, tears, etc but you also need to drink water to keep hydrated and keep your water levels the same so that isn't it. You breath in oxygen to carry out these oxidative reactions and you breath out carbon dioxide as a waste product. That isn't balanced, carbon dioxide is essentially oxygen plus carbon. So if you breath in 6 oxygen molecules and breath out 6 carbon dioxide molecules you have essentially lost 6 carbons from your body.
Now if all you do is burn the glucose you eat for energy then that carbon comes from the glucose you ate so you would not lose any weight (the C you breath out is balanced by the C you just ate). However if you take in less calories than you need to power your body you will break down glycogen (stored glucose) and/or fats (stored hydrocarbons) within your body by oxidizing them from breathed in oxygen and then breath out carbon dioxide. As a result, you lose weight. If, however, you eat more calorically than your body requires for energy your body will store the excess in the form of either glycogen (for glucose) or triglycerides (for fats) and since those molecules have weight you will gain weight. Although I used glucose as the example here the sample principle would hold true of fats as well. Proteins also contain nitrogen and waste nitrogen is excreted in the form of uric acid in our urine.
So what is the answer? When you lose weight the weight you are losing is being breathed out in the form of carbon in carbon dioxide. The amount of carbon dioxide you breath out is connected to how metabolically active you are which in turn is connected to how physically active you are being. If you go for a run you will breakdown molecules to create energy which will require more oxygen and expel more carbon dioxide which is why you have to breath harder. If, instead, you just sit on your couch and breath hard you are taking more breaths but you aren't actually producing more carbon dioxide all you are doing is expelling less carbon dioxide per exhale...that is just hyperventilating and won't do anything other than making you a bit light headed.
You could write a biochemistry textbook. Wow!
I taught a section of upper division biochem at UW for a year, but haven't written any textbooks. Am published though.5 -
Ok... I get that 02 =/= CO2 in terms of weight. So some is leaving <bye bye>!!!
How come ATP = ADP? Or are we just rounding there?
Good question, it is because I didn't explicitly write out the phosphate group. ATP = ADP + P in terms of weight and neither leave your body, they are just no longer bonded together.
Picture it like a rechargable battery that stays in your body. ATP is charged, ADP + P is discharged. When the bond in ATP between ADP and P is broken energy is released that can be used, but the P doesn't go anywhere it is just floating around until it gets re-bonded to ADP for a new "charge" to ATP.
That is why I went with a rechargeable battery analogy. A charged battery weighs the same as a discharged battery. ATP weighs the same as ADP+P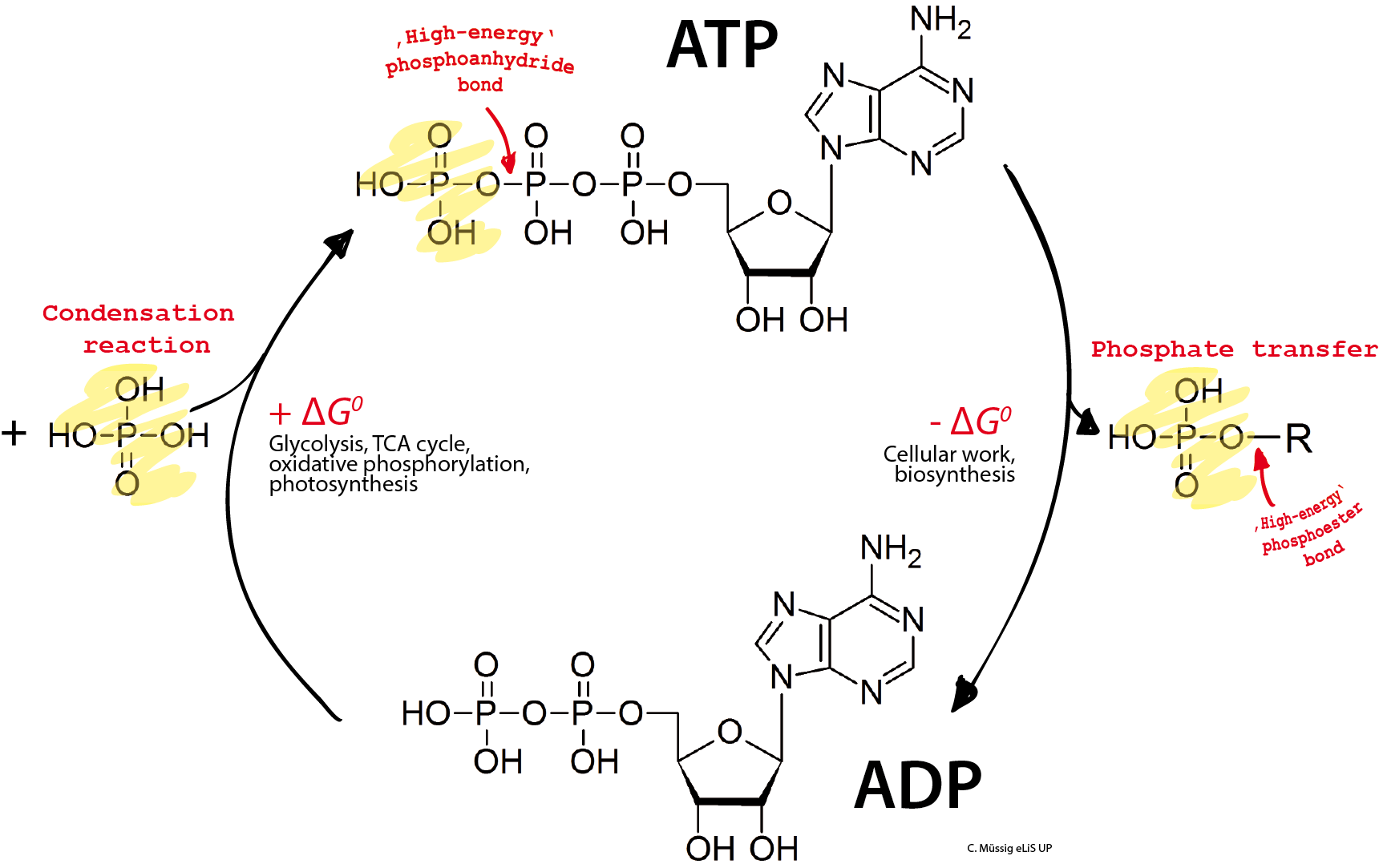 4
4
Categories
- All Categories
- 1.4M Health, Wellness and Goals
- 398.1K Introduce Yourself
- 44.7K Getting Started
- 261K Health and Weight Loss
- 176.4K Food and Nutrition
- 47.7K Recipes
- 233K Fitness and Exercise
- 462 Sleep, Mindfulness and Overall Wellness
- 6.5K Goal: Maintaining Weight
- 8.7K Goal: Gaining Weight and Body Building
- 153.5K Motivation and Support
- 8.4K Challenges
- 1.4K Debate Club
- 96.5K Chit-Chat
- 2.6K Fun and Games
- 4.8K MyFitnessPal Information
- 18 News and Announcements
- 21 MyFitnessPal Academy
- 1.5K Feature Suggestions and Ideas
- 3.2K MyFitnessPal Tech Support Questions


















