A couple of things I don't understand about weight loss
Replies
-
Wow, thanks, that makes a lot of sense! Just one more question, for all you science guys out there. Why doesn't weightlifting burn calories? You would think that you're working your muscles, so the same thing should happen...
I feel that I am definitely burning more calories when I lift weights than when I am sitting at my computer and so log it in my exercise tab under the cardio section using the entry "Weight training, free weights." I don't eat 100% of the calories I earned back.
Some people chose to not log weighlifting.
/shrug/0 -
EvgeniZyntx wrote: »This is basically the building blocks of fat...
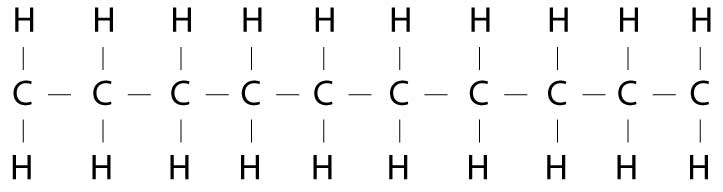
Carbon and hydrogen (toss in a few oxygens and other stuff but 99% carbon and oxygen)
As has been mentioned the carbon, once broken down goes out as CO2. And the hydrogen is also mixed with oxygen to make water. Excreted or used in other processes. Well, partially true.
Those carbons are also used to make cholesterol (all your cells have walls from fat) and every hormone and signaling molecule and glucose molecule, etc, etc, made by your liver comes mostly from fat stores. No fat... no life.
Yeah, what oxygen there is typically comes in the carboxylic acid at the end, hence fatty acid.
Oh and while we are geeking out over biochemistry to anyone reading, see how every carbon has two hydrogens attached to it? That is what makes it a saturated fat. If instead in that chain one of the bonds is a double bond to the neighboring carbon it will have only one hydrogen attached to it. That will additionally make the chain kink and with that kink the molecules don't pack together as well. That is why typically saturated fats will be solid at room temp while unsaturated will be an oil.
Fat typically is stored as a triglyceride...three fatty acid chains attached to a molecule of glycerol. Here is an example of one with two saturated chains and one unsaturated chain. 2
2 -
/enters thread
Sees discussion about carbon bonds, including diagrams.
/exits thread5 -
-
quiksylver296 wrote: »
Hah I think she is exiting because it's taken care of not because she has a fear of chemistry. Just a guess
Still...even when you know your Chem still a good fun watch, very good talk so yeah recommend.2 -
quiksylver296 wrote: »Aaron_K123 wrote: »quiksylver296 wrote: »
Hah I think she is exiting because it's taken care of not because she has a fear of chemistry. Just a guess
Still...even when you know your Chem still a good fun watch, very good talk so yeah recommend.
Today it's more of a "I'm too sick of chemistry for this" type of thread-exit. But I'll definitely check the vid out later.2 -
quiksylver296 wrote: »Aaron_K123 wrote: »quiksylver296 wrote: »
Hah I think she is exiting because it's taken care of not because she has a fear of chemistry. Just a guess
Still...even when you know your Chem still a good fun watch, very good talk so yeah recommend.
Today it's more of a "I'm too sick of chemistry for this" type of thread-exit. But I'll definitely check the vid out later.
That was my second guess. Can only hear the word stoicheometry so many times before you punch someone I imagine. Do come back though, good video and you likely have good insight to add.1 -
This has been one of the most educational threads I've ever seen on MFP. Very interesting stuff.1
-
This content has been removed.
-
This is a hugely educational thread! Thank you so much!0
-
Do the difference between cis and trans fats.1
-
-
Love this thread, everyone. Thanks!0
-
Can always count on you to give an understandable answer. Nice job bro.Aaron_K123 wrote: »You breath in oxygen. O2. You breath out carbon dioxide. CO2. That carbon atom is where your weight is lost, in that exchange, through respiration.
The answer to "where does a plants mass come from?" Is the same answer in reverse.
Yes I know there is also nitrogen exchange but carbon from respiration is the primary source.
So short answer...where does the weight go? You breath it out.
Fancier answer food is primarily hydrocarbons with some nitrogen. The oxygen and hydrogen that aren't incorporated into your own macromolecules are formed into H20, water, and excreted in urine. Nitrogen waste is converted to uric acid and also goes out as waste. Undigestables go out as solid waste. Carbon waste goes out via your breath.
Fat is pure hydrocarbon, no nitrogen. You burn fat you convert it to water which you excrete and carbon you breath out.
A.C.E. Certified Personal and Group Fitness Trainer
IDEA Fitness member
Kickboxing Certified Instructor
Been in fitness for 30 years and have studied kinesiology and nutrition
0 -
This would be a great topic to have in the helpful thread announcement.0
-
Do the difference between cis and trans fats.
Best explanation I've seen:
indiana.edu/~oso/Fat/trans.html0 -
Do the difference between cis and trans fats.
Not sure if serious but why not but what the heck.
In cis the carbon atoms that are linked to the double bonded carbon atoms are on the same side of the double bond which in terms of a fatty acid chain creates a substantial kink and the chain turns in the direction of the carbons.
In trans the linked carbons are on the opposite side which causes a very small kink and the chain remains pretty much straight. The effect of this is that trans unsaturated fats act mechanically more like saturated fat (ie solid at room temp).
I believe cis is the form that occurs in living organisms but if you unsaturate fat chemically rather than enzymatically you get both types.
2 -
Also, there are small amounts of naturally occurring trans fatty acids in meat and dairy products, but it is unknown if they pose the same health problems as manufactured trans fats.0
-
"How does weight actually "get lost." if you exercise, you lose pounds. so your steak and the rest will be consumed, and add calories, makes you gain weight...but when you exercise you burn off the weight.
"How does weight "get gained"? ...the body is an ever growing, ever evolving organism thats not linear. there are many reasons for your pound and a half to come on overnight, like water retention. if you exercised at all, it could be increased muscle mass.
"What does exercise do?" exercise heats up your body, and when that happens you burn fat. (fat = weight)0 -
"How does weight actually "get lost." if you exercise, you lose pounds. so your steak and the rest will be consumed, and add calories, makes you gain weight...but when you exercise you burn off the weight.
"How does weight "get gained"? ...the body is an ever growing, ever evolving organism thats not linear. there are many reasons for your pound and a half to come on overnight, like water retention. if you exercised at all, it could be increased muscle mass.
"What does exercise do?" exercise heats up your body, and when that happens you burn fat. (fat = weight)
So what are you saying then, that our bodies vaporize fat by heating it and exercise literally burns fat because when you exercise your body becomes hotter? If so then no, not at all.
Basically you have it backwards, heat isn't used to metabolize fat, heat is a biproduct of fat (and other hydrocarbon) metabolism. Our body temperature is above ambient and is maintained through release of heat from these metabolic processes. Much of the calories used in your BMR are in the maintenance of your body temperature. In fact the fact that these metabolic processes run most efficiently at 37c (98.6F) is why our bodies are kept warm in the first place. Heating above that will if anything hinder, not help.
If it was the opposite, that fat metabolism requires heat rather than producing it, where do you think our body heat comes from?2 -
"How does weight actually "get lost." if you exercise, you lose pounds. so your steak and the rest will be consumed, and add calories, makes you gain weight...but when you exercise you burn off the weight.
"How does weight "get gained"? ...the body is an ever growing, ever evolving organism thats not linear. there are many reasons for your pound and a half to come on overnight, like water retention. if you exercised at all, it could be increased muscle mass.
"What does exercise do?" exercise heats up your body, and when that happens you burn fat. (fat = weight)
So you're saying we're basically incenerators and the fat just melts away because our bodies get so hot from exercising?
Not quite.0 -
Aaron_K123 wrote: »Do the difference between cis and trans fats.
Not sure if serious but why not but what the heck.
In cis the carbon atoms that are linked to the double bonded carbon atoms are on the same side of the double bond which in terms of a fatty acid chain creates a substantial kink and the chain turns in the direction of the carbons.
In trans the linked carbons are on the opposite side which causes a very small kink and the chain remains pretty much straight. The effect of this is that trans unsaturated fats act mechanically more like saturated fat (ie solid at room temp).
I believe cis is the form that occurs in living organisms but if you unsaturate fat chemically rather than enzymatically you get both types.
Very interesting, thank you.
Do animal saturates and plant saturates have the same straight atom feature?0 -
This is really interesting, thank you all! I think I need to read it a few times to fully grasp the chemistry, but it's a fascinating read. (Count me as another who never actually thought about this).0
-
Agreed with the rest. This is an awesomely educational thread. Never really thought of the biochemistry involved, but you guys (and the vid) explained it so clearly!0
-
RuNaRoUnDaFiEld wrote: »Aaron_K123 wrote: »Do the difference between cis and trans fats.
Not sure if serious but why not but what the heck.
In cis the carbon atoms that are linked to the double bonded carbon atoms are on the same side of the double bond which in terms of a fatty acid chain creates a substantial kink and the chain turns in the direction of the carbons.
In trans the linked carbons are on the opposite side which causes a very small kink and the chain remains pretty much straight. The effect of this is that trans unsaturated fats act mechanically more like saturated fat (ie solid at room temp).
I believe cis is the form that occurs in living organisms but if you unsaturate fat chemically rather than enzymatically you get both types.
Very interesting, thank you.
Do animal saturates and plant saturates have the same straight atom feature?
Yes, saturated fat is saturated fat. It does not matter whether it is from an animal or a plant source. It will be a straight carbon chain filled (saturated) with all the hydrogen possible. Most fats are a mixture of saturated and unsaturated (monounsaturated and polyunsaturated).1
This discussion has been closed.
Categories
- All Categories
- 1.4M Health, Wellness and Goals
- 398.4K Introduce Yourself
- 44.7K Getting Started
- 261K Health and Weight Loss
- 176.4K Food and Nutrition
- 47.7K Recipes
- 233K Fitness and Exercise
- 462 Sleep, Mindfulness and Overall Wellness
- 6.5K Goal: Maintaining Weight
- 8.7K Goal: Gaining Weight and Body Building
- 153.5K Motivation and Support
- 8.4K Challenges
- 1.4K Debate Club
- 96.5K Chit-Chat
- 2.6K Fun and Games
- 4.7K MyFitnessPal Information
- 17 News and Announcements
- 21 MyFitnessPal Academy
- 1.5K Feature Suggestions and Ideas
- 3.2K MyFitnessPal Tech Support Questions














