Why Aspartame Isn't Scary

Aaron_K123
Posts: 7,122 Member
Hey everyone. I've seen my fair share of posts on the forums with regards to the dangers of aspartame and how it is a poison or a toxin or a carcinogen. Wanted to clear some things up about aspartame if I could just to explain why I personally believe there is absolutely no reason to fear aspartame.
What is aspartame?
For my fellow biochemists just simply saying its a methylester of phenylalanine and aspartate is enough to answer that question but figure I should take the time to explain what that means. Phenylalanine and aspartate are 2 of the 20 naturally occuring amino acids found in all protein. As our sequence information databases grow we know more and more about what the average amino acid composition of proteins is. Here is a download of our sum total sequence information from protein from the UniProt database. https://web.expasy.org/docs/relnotes/relstat.html Section 6 shows the amino acid frequencies which show phenylalanine (Phe, F) at 3.6% of protein and aspartate (Asp, D) as 5.46%. This information will come in handy later. Amino acids are connected to one another naturally via a peptide bond between the carboxylic acid group and the amino group of each individual amino acid. Aspartame is simply a dipeptide of phenylalanine and aspartate where the terminal carboxyl group is substituted for a methyl ester.
All amino acids have the following structure:

Aspartame's structure is this:
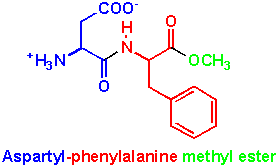
And the natural dipeptide between aspartate and phenylalanine (aspartyl-phenylalanine) is this:

Aspartame's structure is just a natural dipeptide of phenylalanine and aspartate where the terminal carboxylic acid group has been methylated on the oxygen to form a methyl ester so instead of COO- it is COCH3. That is the only difference.
What happens to aspartame when we ingest it?
As with any protein, aspartame is hydrolyzed in the stomach acid and metabolically broken down in the intestine to the breakdown products of aspartame, phenylalanine, and methanol in a weight ratio of 4:5:1. What that means is that 10mg of aspartame will be broken down in your body to 4mg of aspartate, 5mg of phenylalanine, and 1mg of methanol before it enters your blood. No aspartame enters your blood intact.
How much of each metabolite do you get from ingesting one diet soda?
So the metabolic products of aspartame are aspartate,phenylalanine, and methanol in a 4:5:1 ratio. One can of diet coke has about 180mg of aspartame. That means it is broken down to 72mg of aspartate, 90mg of phenylalanine, and 18mg of methanol.
How much of those metabolites are in other foods?
As mentioned phenylalanine and aspartate are naturally occurring amino acids found in all proteins. Protein is about 5.46% aspartate and about 3.6% phenylalanine on average. So let us say you have a 4oz piece of chicken breast. A small 4oz chicken breast has about 24g of protein. That means that in that chicken breast there is .036*24*1000 = 864mg of phenylalanine and .055*24*1000 = 1320mg aspartate. That means to get the same amount of aspartate and phenylalanine from diet coke as you do from one 4oz chicken breast you would have to drink 18 diet cokes. In my diet I eat around 180g of protein in a day which means to equal the amount I get from my normal diet of whole foods I would have to drink 135 cans of diet coke.
Methanol is a bi-product of all fermentations. As such it is present in things that ferment, including things that are in the process of fermenting whether we think of them as alcohol or not. That means things like fruit. So how much methanol is present in 1 8oz glass of orange juice for example? Well according to this study of the presence of methanol in a variety of orange juices [citation: http://archive.food.gov.uk/maff/archive/food/infsheet/1993/no17/table1.htm] the amount of methanol averages around 125 mg/kg. 8oz is 0.23kg so that means that 8oz of orange juice has about 29mg of methanol in it. Recall that in a diet soda the aspartame content would break down to about 18mg of methanol. In other words orange juice, or really any fruit juice, has more methanol in it per oz than soda.
Conclusion
We know what aspartame is, we know its structure, we know its composition and we know exactly what happens to it in the human body. We are very familiar with the metabolic breakdown products of phenylalanine, aspartate and methanol all of which are found in higher amounts in natural whole foods such as fruits and proteins. There is no reason at all to suspect that aspartame presents any sort of toxic or carcinogenic risk from the chemistry of the molecule and indeed toxicology studies of aspartame in humans show no toxic dose level [citation: http://informahealthcare.com/doi/abs/10.1080/10408440701516184]. Stories of the toxicity of aspartame are hearsay, anecdotal and fear-mongering and are not supported by either chemistry, biochemistry, toxicology or the epidemiology.
Yet online on the internet, we get stuff like this:

Sensationalistic irrational garbage.
What is aspartame?
For my fellow biochemists just simply saying its a methylester of phenylalanine and aspartate is enough to answer that question but figure I should take the time to explain what that means. Phenylalanine and aspartate are 2 of the 20 naturally occuring amino acids found in all protein. As our sequence information databases grow we know more and more about what the average amino acid composition of proteins is. Here is a download of our sum total sequence information from protein from the UniProt database. https://web.expasy.org/docs/relnotes/relstat.html Section 6 shows the amino acid frequencies which show phenylalanine (Phe, F) at 3.6% of protein and aspartate (Asp, D) as 5.46%. This information will come in handy later. Amino acids are connected to one another naturally via a peptide bond between the carboxylic acid group and the amino group of each individual amino acid. Aspartame is simply a dipeptide of phenylalanine and aspartate where the terminal carboxyl group is substituted for a methyl ester.
All amino acids have the following structure:

Aspartame's structure is this:

And the natural dipeptide between aspartate and phenylalanine (aspartyl-phenylalanine) is this:

Aspartame's structure is just a natural dipeptide of phenylalanine and aspartate where the terminal carboxylic acid group has been methylated on the oxygen to form a methyl ester so instead of COO- it is COCH3. That is the only difference.
What happens to aspartame when we ingest it?
As with any protein, aspartame is hydrolyzed in the stomach acid and metabolically broken down in the intestine to the breakdown products of aspartame, phenylalanine, and methanol in a weight ratio of 4:5:1. What that means is that 10mg of aspartame will be broken down in your body to 4mg of aspartate, 5mg of phenylalanine, and 1mg of methanol before it enters your blood. No aspartame enters your blood intact.
How much of each metabolite do you get from ingesting one diet soda?
So the metabolic products of aspartame are aspartate,phenylalanine, and methanol in a 4:5:1 ratio. One can of diet coke has about 180mg of aspartame. That means it is broken down to 72mg of aspartate, 90mg of phenylalanine, and 18mg of methanol.
How much of those metabolites are in other foods?
As mentioned phenylalanine and aspartate are naturally occurring amino acids found in all proteins. Protein is about 5.46% aspartate and about 3.6% phenylalanine on average. So let us say you have a 4oz piece of chicken breast. A small 4oz chicken breast has about 24g of protein. That means that in that chicken breast there is .036*24*1000 = 864mg of phenylalanine and .055*24*1000 = 1320mg aspartate. That means to get the same amount of aspartate and phenylalanine from diet coke as you do from one 4oz chicken breast you would have to drink 18 diet cokes. In my diet I eat around 180g of protein in a day which means to equal the amount I get from my normal diet of whole foods I would have to drink 135 cans of diet coke.
Methanol is a bi-product of all fermentations. As such it is present in things that ferment, including things that are in the process of fermenting whether we think of them as alcohol or not. That means things like fruit. So how much methanol is present in 1 8oz glass of orange juice for example? Well according to this study of the presence of methanol in a variety of orange juices [citation: http://archive.food.gov.uk/maff/archive/food/infsheet/1993/no17/table1.htm] the amount of methanol averages around 125 mg/kg. 8oz is 0.23kg so that means that 8oz of orange juice has about 29mg of methanol in it. Recall that in a diet soda the aspartame content would break down to about 18mg of methanol. In other words orange juice, or really any fruit juice, has more methanol in it per oz than soda.
Conclusion
We know what aspartame is, we know its structure, we know its composition and we know exactly what happens to it in the human body. We are very familiar with the metabolic breakdown products of phenylalanine, aspartate and methanol all of which are found in higher amounts in natural whole foods such as fruits and proteins. There is no reason at all to suspect that aspartame presents any sort of toxic or carcinogenic risk from the chemistry of the molecule and indeed toxicology studies of aspartame in humans show no toxic dose level [citation: http://informahealthcare.com/doi/abs/10.1080/10408440701516184]. Stories of the toxicity of aspartame are hearsay, anecdotal and fear-mongering and are not supported by either chemistry, biochemistry, toxicology or the epidemiology.
Yet online on the internet, we get stuff like this:

Sensationalistic irrational garbage.
153
Replies
-
I notice if I eat too much this "sugar free" stuff, I have 2 problems: craving and bloating...not sure if it's any direct correlation though.24
-
OP, :drinker:9
-
Bump to read later.3
-
Very interesting and well explained (albeit in a uber-scientific way)
So then just out of curiosity, what is it - scientifically speaking - that was found in the studies some rely on to "prove" aspartame is harmful. Is it a super-duper concentration (un-scientifically speaking) of one of the specific compounds? That's my suspicion, but I'd be interested in an explanation from someone far smarter than myself just what it is the anti-aspartame folks are hanging their hat on (the ones who actually sort of know what they're talking about; not the ones whose totality of 'informed' opinion comes from forwarded emails and facebook posts)6 -
Aspartame has never scared me. I will never stop drinking Diet Pepsi. Never!
http://www.ncbi.nlm.nih.gov/pubmed/17828671
http://www.cancer.org/cancer/cancercauses/othercarcinogens/athome/aspartame22 -
Very interesting and well explained (albeit in a uber-scientific way)
So then just out of curiosity, what is it - scientifically speaking - that was found in the studies some rely on to "prove" aspartame is harmful. Is it a super-duper concentration (un-scientifically speaking) of one of the specific compounds? That's my suspicion, but I'd be interested in an explanation from someone far smarter than myself just what it is the anti-aspartame folks are hanging their hat on (the ones who actually sort of know what they're talking about; not the ones whose totality of 'informed' opinion comes from forwarded emails and facebook posts)
I honestly don't think people who scare-monger against aspartame have any legitimate evidence of toxicity of any level.
As stated we know the metabolic breakdown products of aspartame. They are aspartate and phenylalanine, amino acids that are non-toxic unless you have PKU and methanol. Methanol gets further converted to formaldehyde which does have toxicity which is why methanol is toxic. But with all things toxic there is a dose at which they are toxic and below which they are not.
The toxic dose of methanol far far FAR exceeds the amount you get in a soda or in fruit juices or in wine.
To answer your question as best I can let me put it this way. If you were to drink diet soda, one right after the other non-stop until you died the substance that killed you would be water. Water is therefore the most "toxic" ingredient in diet soda.40 -
I... can I hug you? Can we be friends? Unless your body has issues processing phenylalanine (my grandmother, for instance), it's nothing to be afraid of. It's like everything else... don't consume it in excess and you should be just peachy.
Edited because words. Spelling.27 -
Very interesting and well explained (albeit in a uber-scientific way)
So then just out of curiosity, what is it - scientifically speaking - that was found in the studies some rely on to "prove" aspartame is harmful. Is it a super-duper concentration (un-scientifically speaking) of one of the specific compounds? That's my suspicion, but I'd be interested in an explanation from someone far smarter than myself just what it is the anti-aspartame folks are hanging their hat on (the ones who actually sort of know what they're talking about; not the ones whose totality of 'informed' opinion comes from forwarded emails and facebook posts)
I honestly don't think people who scare-monger against aspartame have any legitimate evidence of toxicity of any level.
As stated we know the metabolic breakdown products of aspartame. They are aspartate and phenylalanine, amino acids that are non-toxic unless you have PKU and methanol. Methanol gets further converted to formaldehyde which does have toxicity which is why methanol is toxic. But with all things toxic there is a dose at which they are toxic and below which they are not.
The toxic dose of methanol far far FAR exceeds the amount you get in a soda or in fruit juices or in wine.
To answer your question as best I can let me put it this way. If you were to drink diet soda, one right after the other non-stop until you died the substance that killed you would be water. Water is therefore the most "toxic" ingredient in diet soda.
That's basically what I suspected.
I'm going all-idiot now and do exactly what I ridiculed others for doing...I "heard" there were studies that showed that super-high doses of aspartame caused cancer in mice. Never been too worried about it to dig up the research or anything...I just suspected that it was ultra high doses of one of the component chemicals that would require chugging gallons of the stuff. But was just curious for a scientific explanation if it was different than my supposition.
I don't drink soda - but only because I don't particularly care for it (except in small doses mixed with some whiskey or rum or something). I do tend to search out products (yogurts, etc) that have sugar vs. aspartame because I simply like the taste better.
Appreciate the research and explanation!3 -
Very interesting and well explained (albeit in a uber-scientific way)
So then just out of curiosity, what is it - scientifically speaking - that was found in the studies some rely on to "prove" aspartame is harmful. Is it a super-duper concentration (un-scientifically speaking) of one of the specific compounds? That's my suspicion, but I'd be interested in an explanation from someone far smarter than myself just what it is the anti-aspartame folks are hanging their hat on (the ones who actually sort of know what they're talking about; not the ones whose totality of 'informed' opinion comes from forwarded emails and facebook posts)
I honestly don't think people who scare-monger against aspartame have any legitimate evidence of toxicity of any level.
As stated we know the metabolic breakdown products of aspartame. They are aspartate and phenylalanine, amino acids that are non-toxic unless you have PKU and methanol. Methanol gets further converted to formaldehyde which does have toxicity which is why methanol is toxic. But with all things toxic there is a dose at which they are toxic and below which they are not.
The toxic dose of methanol far far FAR exceeds the amount you get in a soda or in fruit juices or in wine.
To answer your question as best I can let me put it this way. If you were to drink diet soda, one right after the other non-stop until you died the substance that killed you would be water. Water is therefore the most "toxic" ingredient in diet soda.
That's basically what I suspected.
I'm going all-idiot now and do exactly what I ridiculed others for doing...I "heard" there were studies that showed that super-high doses of aspartame caused cancer in mice. Never been too worried about it to dig up the research or anything...I just suspected that it was ultra high doses of one of the component chemicals that would require chugging gallons of the stuff. But was just curious for a scientific explanation if it was different than my supposition.
I don't drink soda - but only because I don't particularly care for it (except in small doses mixed with some whiskey or rum or something). I do tend to search out products (yogurts, etc) that have sugar vs. aspartame because I simply like the taste better.
Appreciate the research and explanation!
The study you are refering to are two studies conducted by the same lab Soffritti et al 2006 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1964906/ and Soffritti et al 2007 located here for the full article http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1964906/
The studies were conducted in rats, specifically Sprague-Dawley rats. This is a rather odd and questionable choice of model organism. Why it is questionable is that really Sprague-Dawley rats are the model organism of choice for studying cancer because they are outbred rats who are known to develop spontaneous tumors 45% of the time. http://cancerres.aacrjournals.org/content/33/11/2768.full.pdf
Basically you pick Sprague Dawly rats if you want some rats that are going to develop tumors no matter what. The fact that this groups Sprague-Dawley rats developed tumors is therefore no suprise at all and doesn't mean a thing about what affect aspartame had on them.
So I have to ask why, if these researchers wanted to test if a particular compound was carcinogenic, would they EVER choose to test it in Sprague-Dawley rats...rats known for forming spontaneous tumors due to their genetics. Toxicology testing is routinely done in BALB/c or C57B/6 mice so the use of Sprague-Dawley rats is odd.
Also I have to point out that if aspartame made 40% of those who ingest it break out all over the place in tumors I think we would have noticed by now.
It is one flawed study amongst hundreds of studies that demonstrate no carcinogenic or toxic effects of aspartame and yet it is the one study cited over and over by articles talking about how "toxic" aspartame is. Frankly I doubt those people have ever read the study or know anything about Sprague-Dawley rats.45 -
Also on a side note "toxin" and "carcinogen" refer to two completely separate concepts. When someone talks about how something is a "toxin" and then links to a study about it causing cancer I just have to facepalm. It is an immediate indicator though that they really don't know what they are talking about.
A toxin is a poison which basically means that at a certain dose it is fatal or damaging. A carcinogen is something that increases the odds of developing cancer. Not all carcinogens are toxins, not all toxins are carcinogens...they are two wholly separate things.
Aspartame, as it happens, is neither a toxin or a carcinogen.30 -
Science is so awesome!
Appreciate the info!9 -
Thanks!0
-
In case it is relevant my background is a Ph.D. in molecular biology with a focus on protein biochemistry. 9 years of research experience with 5 years of infectious disease drug development research part of which is performing toxicology assays on potential drugs. In my opinion there is no evidence or reason to suspect mechanistically that aspartame is toxic or carcinogenic.
I do NOT think that expertise or background is proof that someone is correct so I invite anyone and everyone who is interested to look into this yourself. I just mention it because I know I will likely be asked.40 -
I do NOT think that expertise or background is proof that someone is correct so I invite anyone and everyone who is interested to look into this yourself. I just mention it because I know I will likely be asked.
Agreed - which is why I was curious as to your analysis of the anti-aspartame studies.
I'd be equally interested in someone who is anti-aspartame to explain exactly why they feel that way...but fair warning "because I read it on the internet" or "because it's hard to pronounce" will not be acceptable answers over science.5 -
I do NOT think that expertise or background is proof that someone is correct so I invite anyone and everyone who is interested to look into this yourself. I just mention it because I know I will likely be asked.
Agreed - which is why I was curious as to your analysis of the anti-aspartame studies.
I'd be equally interested in someone who is anti-aspartame to explain exactly why they feel that way...but fair warning "because I read it on the internet" or "because it's hard to pronounce" will not be acceptable answers over science.
I also encourage anyone who honestly believe that aspartame is dangerous to post here and explain in a reasoned way why you feel that is the case.2 -
Why even risk it? After all, it still is a possible carcinogen. I stay away from all artificial sweeteners. I only eat the real stuff.14
-
Why even risk it? After all, it still is a possible carcinogen. I stay away from all artificial sweeteners. I only eat the real stuff.
No...it isn't a carcinogen. What do you mean by saying its a "possible carcinogen". What isn't a "possible carcinogen" by your definition?
Aspartame is plenty real so not sure what you mean by "real stuff" either.
Why even risk it? Why live in fear for no reason would be my question.18 -
Why even risk it? After all, it still is a possible carcinogen. I stay away from all artificial sweeteners. I only eat the real stuff.
...and there you have it. Conclusive proof.
I can possibly get cancer by going on a long run if I forget to put on sunscreen or accidentally inhale too many diesel fumes. Guess it's best to just sit on my couch all day.
What I'd like to know is what about it/how it is possibly carcinogenic. You know...scientifically speaking.9 -
Why even risk it? After all, it still is a possible carcinogen. I stay away from all artificial sweeteners. I only eat the real stuff.
No...it isn't a carcinogen. What do you mean by saying its a "possible carcinogen". What isn't a "possible carcinogen" by your definition?
Aspartame is plenty real so not sure what you mean by "real stuff" either.
Why even risk it? Why live in fear for no reason would be my question.
Well in fairness, you did explicitly ask for beliefs and feelings, as opposed to evidence and critical thinking. While the two might sometimes end up in the same place, they have nothing to do with each other as a means of dealing with the world.2 -
Oh wow! Very informative. I had been wondering about aspartame myself. A guy I know said he cut out aspartame and sucralose and what he calls "fake sugars" and claimed to lose 40 pounds in a month. (He was about 290.) I didn't tell him this, but I think he lost that weight because he cut way down on calories, but he attributed the loss to the cutting out of fake sugars instead.9
-
Oh wow! Very informative. I had been wondering about aspartame myself. A guy I know said he cut out aspartame and sucralose and what he calls "fake sugars" and claimed to lose 40 pounds in a month. (He was about 290.) I didn't tell him this, but I think he lost that weight because he cut way down on calories, but he attributed the loss to the cutting out of fake sugars instead.
And if he weighed the same as a duck, he'd be made of wood. Thus, we could burn him as a witch...if we were in the business of burning witches, that is.23 -
There has been too much debate over whether or not artificial sweeteners can cause disease, so I simply stay away. Makes sense to me.5
-
Yeah, Mr. White, science!
This is a great post, thanks!8 -
Why even risk it? After all, it still is a possible carcinogen. I stay away from all artificial sweeteners. I only eat the real stuff.
The only thing arguably toxic in the byproducts of aspartame is methanol which you can find much more of in fruit than in a can of diet soda. So unless you are avoiding fruit because "why risk it?" I have to question your logic there.11 -
There has been too much debate over whether or not artificial sweeteners can cause disease, so I simply stay away. Makes sense to me.
So...then...by your words you would then be avoiding pretty much every food available...which was the point of his thread. All the makings of Aspartame all ready happen naturally in all the food you eat.4 -
There has been too much debate over whether or not artificial sweeteners can cause disease, so I simply stay away. Makes sense to me.
Same like how there is lots of "debate" about evolution so best not to "believe" in it amiright?
There is no "debate" about the toxicity or carcinogenicity of aspartame. There are the facts and there are people making blog posts on the internet saying "why risk it?"9 -
Why even risk it? After all, it still is a possible carcinogen. I stay away from all artificial sweeteners. I only eat the real stuff.
The only thing arguably toxic in the byproducts of aspartame is methanol which you can find much more of in fruit than in a can of diet soda. So unless you are avoiding fruit because "why risk it?" I have to question your logic there.
It isn't logical. It's pascal's wager. Whether or not that's a problem is a larger question.1 -
All I know is that artificial sweeteners (not just aspartame, but sucrolose, sorbitol, xylitol, and some others as well) all make me feel yucky. And I'm not talking big quantities either: one squirt of Mio in my water, one piece of sugar-free chewing gum. I get bloated, and a little nauseous. More than that can lead to some intense headaches. Years ago, I managed to quit drinking soda by switching to diet. It made me sick enough to avoid it all for good.
Nectresse is one that doesn't seem to bother me.
Maybe I'm just more sensitive to it. I'm a big fan of the N=1 experiment. If you're curious if a food is good or bad for you, cut it out completely for about a month. Then try some and pay attention to how you feel afterwards. You might notice a difference, you might not.8 -


Can no longer edit my original post so here is a graphic of the metabolic breakdown of aspartame into phenylalanine, aspartate and methanol.
Having real issues to get those images to resize to look good here but that last one is its breakdown to those three metabolites and the first one is where those metabolites come from in the original molecule.1 -
There has been too much debate over whether or not artificial sweeteners can cause disease, so I simply stay away. Makes sense to me.
So you just avoid anything that anyone debates over?
Good heavens, girl. What do you possibly eat??
Meat? No some vegans debate that. Better cross it off the list.
Dairy? Good heavens no.
Sugar? Egads.
Non-organic produce? Forget about it.
Organic Produce? Nuh-uh
Carbs? Wrong answer19
Categories
- All Categories
- 1.4M Health, Wellness and Goals
- 398.2K Introduce Yourself
- 44.7K Getting Started
- 261K Health and Weight Loss
- 176.4K Food and Nutrition
- 47.7K Recipes
- 233K Fitness and Exercise
- 462 Sleep, Mindfulness and Overall Wellness
- 6.5K Goal: Maintaining Weight
- 8.7K Goal: Gaining Weight and Body Building
- 153.5K Motivation and Support
- 8.4K Challenges
- 1.4K Debate Club
- 96.5K Chit-Chat
- 2.6K Fun and Games
- 4.8K MyFitnessPal Information
- 12 News and Announcements
- 21 MyFitnessPal Academy
- 1.6K Feature Suggestions and Ideas
- 3.2K MyFitnessPal Tech Support Questions












